
Aminoglycosides - Medicinal Chemistry III B. Pharma 6th Semester
Aminoglycosides
Contents
• Introduction
• Chemistry of Aminoglycosides
• Structure and Activity Relationship of Aminoglycosides
• Spectrum of activity of Aminoglycosides
• Uses of single Aminoglycosides / combinations
• Mechanism of Action of Aminoglycosides
• Study of Individual compounds
Learning Objectives
At the end of this lecture, student will be able to
• Understand the chemistry of Aminoglycosides
• Explain the SAR of Aminoglycosides
• Discuss the spectrum of activity of Aminoglycosides
• Explain the general mode of action of Aminoglycosides
• Discuss the structures and specific uses of individual
Aminoglycosides
• Streptomycin 1st aminoglycoside antibiotic used in chemotherapy.
• Other compounds closely related in structure are Kanamycin, Neomycin, Paramomycin, Gentamycin, Tobramycin, Netilmycin and Amikacin (semi synthetic derivative of Kanamycin A)
• All aminoglycoside antibiotics are absorbed very poorly following oral administration yet some of them eg. Kanamycin, Neomycin and paramomycin are administered orally for the treatment of G.I. Infections.
• Due to their broad spectrum anti-microbial activity, they are used to treat systemic infections (parenteral route).
• Side effects include ototoxicity and nephrotoxicity- this has restricted their systemic use to serious infections or to treat infections caused bacterial strains resistant to other agents
Aminoglycosides Chemistry
• Amino-glycosides are amino-sugars linked glycosidically.
• All have at least one aminohexose, or a pentose lacking an amino group eg. Streptomycin, neomycin and paromomycin.
• They contain a highly substituted 1, 3-diaminocyclohexane central ring eg. Kanamycin, neomycin, gentamycin
• They are strongly basic compounds that exist as polycations at physiological pH.
• Their inorganic salts are highly water soluble
• They are available as sulphate salts
• They distribute well into most body fluids but not CNS, bone or fatty connective tissue
• They are concentrated in the kidneys, and excreted by glomerular filtration
• They are not metabolized in vivo
Mechanism of action
• They act directly on the bacterial ribosome to inhibit the initiation of protein synthesis and to interfere with the translation of the genetic messages.
• They bind to the 30S ribosomal subunit to form a complex that cannot initiate proper aminoacid polymerization
• This binding to ribosomes also causes misreading mutations of the genetic code, due to the failure of specific aminoacyl RNA’s to recognize the proper codons on mRNA and hence cause incorporation of improper aminoacids into the peptide chain.
SAR
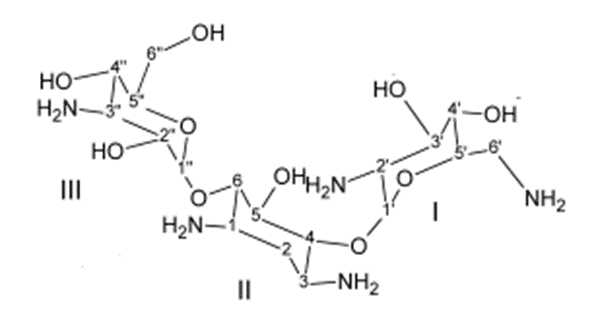
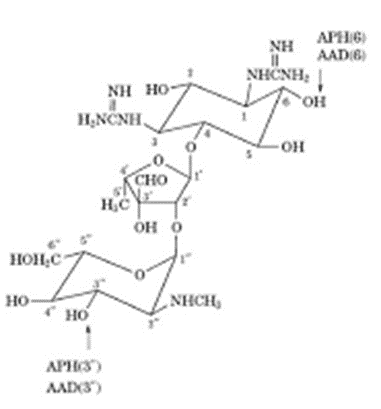
Ring I:-
• Crucially important for characteristic broad spectrum anti-bacterial activity and it is the primary target for bacterial inactivating enzymes.
• Amino functions at 6’ and 2’ are important as Kanamycin – B (6’-amino -2’amino) is more active than Kanamycin – A (6’- Amino- 2’- hydroxyl) which in turn is more active than Kanamycin - C (6’- hydroxyl-2’-amino group)
• Methylation at either the 6’-carbon or 6’-amino positions does not lower anti-bacterial activity, but confers resistance to enzymatic acetylation of the 6’-amino group.
• Removal of the 3’-hydroxyl or the 4’-hydroxyl or both in the Kanamycins (eg., 3’,4’-dideoxy kanamycin B or dibekacin) does not
Ring- II:-
• Few modifications of ring II functional groups is possible without appreciable loss of activity.
• 1-amino group of Kanamycin –A, can be acylated eg. Amikicin and the activity is largely retained.
• Netilmicin retains the antibacterial potency of sisomicin and is resistant to several additional bacteria-inactivating enzymes.
Ring III
• Ring III functional groups are less sensitive to structural changes than those of either Ring I and II
• 2”–deoxy derivatives are less active than their 2”–hydroxyl counterparts (eg. 2”–deoxy gentamycin).
• 2” – amino derivatives (eg. Seldomycins) are highly active.
• The 3” – amino group of Gentamycins may be 1° or 2° with high antibacterial potency.
• The 4”-hydroxyl group maybe axial or equatorial with little change in potency
Spectrum of Activity
• Aminoglycosides are classified as broad – spectrum antibiotics, but they are mostly used in the treatment of serious infections caused by aerobic Gram -ve bacilli. - Aerobic Gram-ve and Gram+ve cocci (exception - staphylococci) are less sensitive. So in these infections β-lactam and other antibiotics are preferred.
• Aminoglycosides and β-lactam antibiotics exert synergetic effect against some bacterial strains- damage to the cell well is believed to increase the penetration of the aminoglycosides into the bacterial cell
• However the two antibiotic types should not be combined in the same solution as they are chemically incompatible
| DRUG/DRUG COMBINATION | USE |
| Streptomycin | In the chemotherapy of tuberculosis, brucellosis, tularemia and Yersinia infections |
| Paramomycin | Chemotherapy of amoebic dysentery |
| Carbenicillin + Gentamycin | Synergistic against Gentamycin- sensitive strains of P-aeruginosa and other Gram –ve bacilli |
| Penicillin G + Streptomycin or Gentamycin or Kanamycin | In the treatment of enterococcal endocarditis |
Damage of the cell wall caused by the β-lactam antibiotic will increase penetration of aminoglycoside into the bacterial cell wall.
Infections:
• Brucellosis –Undulant fever, an intermittent fever caused by organisms (Brucella-bacteria, primarily pathogenic in animals but which may affect man) transmitted in infected milk from cattle and goats.
• Tularemia – An undulant fever (caused by Brucella tularensis) may be infected to man by various insects or by rats. The lymph glands are involved and they may suppurate.
• Bubonic Plague –An acute fever endemic in Asia and Africa. Causative organism – Pasteurella pestis transmitted by the bites of fleas that have derived the infection from diseased rats. (Characterized by buboes)
• Glanders – A contagious disease of horses and asses sometimes communicated to man through a crack in the skin.
• Streptomycin is equally effective in inhibiting initiation and causing misreading.
• Spectinomycin prevents the initiation of protein synthesis but does not cause misreading.
• All are bactericidal except Spectinomycin.
Streptomycin
• The organism that produce streptomycin, streptomyces griseus, also produce other antibiotic compounds– hydroxyl-streptomycin, mannisido-streptomycin and cycloheximide
• Streptomycin is referred to as streptomycin – A and mannisido
• The problem with the use of streptomycin is the early development of resistant strains of bacteria necessitating a change in therapy.
• Other problems are chronic toxicities:-
• Neurotoxic reactions include vertigo, disturbance of equilibrium, diminished auditory perception
• Nephrotoxicity.
• Neomycin (sulfate) [MYCIFRADIN, NEOBIOTIC]
• Kanamycin
• Isolated from
• Streptomyces Kanamycelicus
KANAMYCIN:
Uses:
• In the treatment of infections of the intestinal tract (eg., bacillary dysentery)
• In the treatment of systemic infections from Gram –Ve bacilli (eg., Klebsiella, Proteus, Enterobacter and Serratia spp.)
• Also used for pre-operative antisepsis of the bowel.
Gentamycin: (Sulfate)
• It has a broad spectrum of activity against many common pathogens both gram +Ve and gram -Ve
Uses:-
• It is effective in the treatment of a variety of skin infections especially in the treatment of burns complicated by pseudomonemia.
• Used for serious systemic and genitourinary tract infections caused by gram –Ve bacteria particularly Pseudomonas, Enterobacter and Serratia spp.





0 Comments: