
Local anti-infective agents and Preservatives - Medicinal Chemistry III B. Pharma 6th Semester
Local anti-infective agents and Preservatives
Contents
• Anti-infective agents – classification
• Local Anti-infective agents – Introduction & Classification
• Mode of action of various Local Anti-infective agents
• Synthesis and specific uses of Furazolidone
• Preservatives – Ideal characteristics & types
Learning Objectives
At the end of this lecture, student will be able to
Describe the term “anti-infective agent”
Classify anti-infective agents
Enlist the ideal characteristics of Local anti-infective agents (Germicides) & Preservatives
Classify Germicides & Preservatives
Describe the mode of action of Germicides
Introduction and Classification of anti-infectives
• Anti-infective agents are those agents which are used in the treatment of infectious diseases
• Selective toxicity is the main aim of modern “anti-infective therapy”
Classification:-
• Anti-infective agents may be classified according to a variety of schemes, as the chemical type of the compound the biological property and therapeutic indication
• A combination of these classification schemes is used to classify the anti-infective agents as
Classification of anti-infectives
Ø Local Anti-infective agents:-
• Alcohols - ethanol, isopropyl alcohol
• Phenols - p-chlorophenol, hexachlorophene, resorcinol, hexyl resorcinol
• Halogen – containing compounds- Iodine tincture, Halozone.
• Oxidizing agents – Hydrogen peroxide, Benzoyl peroxide.
• Cationic surfactants – Benzalkonium chloride, Cetyl pyridium chloride
• Dyes – gentian violet, Methyene blue.
• Nitrogen compounds – Nitrofurazone, Furazolidone.
• Mercury compounds – Nitromersol and Thimerosal
Ø Preservatives :-
• p-hydroxy benzoic acid derivatives – Methyl paraben, etc.,
• Miscellaneous compounds as chlorobutanol, etc.,
Ø Anti-fungal agents :-
• Anti-fungal antibiotics – Nystatin, Candicidin, Hamycin, Griseofulvin, Amphotericin – B.
• Synthetic anti-fungal agents
• Substituted imidazoles: Clotrimazole, Metronidazole, Ketoconazole.
• Miscellaneous compounds- Zinc propionate, Sodium Caprylate, Tolnaftate.
Ø Urinary tract anti-infectives :-
• Quinolones – Nalidixic acid, Norfloxacin, Ciprofloxacin, Perfloxacin
• Miscellaneous – Nitrofurantoin.
Ø Anti-tubercular agents:-
• Synthetic anti-tubercular agents- PAS, INH, Ethanbutol, Pyrazinamide, etc.,
• Anti-tubercular antibiotics – Cycloserine, Rifampicin.
Ø Anti-viral agents:- as
• Amantadine HCl, Acyclovir, Zidovudine
• Anti-AIDS – Azathymidine, Suramin.
Ø Anti – protozoal agents:-
• Metronidazole, Iodoquinol, Dimercaprol
Ø Anthelmintics:-
• Diethyl Carbamazine, Mebendazole, Niclosamide
• Anti-scabious & anti-pedicular agents:-
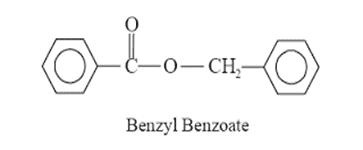
• Benzyl benzoate, Diethyl toluamide, Lindane.
Benzyl Benzoate
Diethyl toluamide
Lindane
Local Anti-infective agents (Germicides)
There are two primary sub-types
Antiseptics:-kill (-cidal) or prevent the growth of (-static) micro-organisms when applied to living tissues.
Ø A useful antiseptic must –
• Have low toxicity so that it can be used directly on skin or wounds.
• Have a rapid and sustained lethal action against micro-organisms.
• Have low surface tension, so that it will spread into the wound.
• Have the ability to retain activity in the presence of body fluids including pus.
• Be non-irritating to tissues & non-allergic
• Lack systemic toxicity when applied to the skin or mucous membrane.
• Have no interference with the healing process of the wound.
Disinfectants:-
Ø A disinfectant is an agent that prevents transmission of infection by the destruction of pathogenic micro-organisms when applied to inanimate objects.
Ø An ideal disinfectant:-
• Exerts a rapid lethal action against all potentially pathogenic microorganisms & spores.
• Have good penetrating properties into organic matter.
• Should be compatible with organic compounds (particularly soaps).
• Is not inactivated by living tissues.
• Is non-corrosive
• Is esthetically pleasing (non-staining or odorless)
Alcohols and related compounds:-
• Antibacterial action is due to their ability to denature the bacterial proteins & inhibit phosphorylation systems
Ethanol
• Clear, Colorless, Volatile liquid, Burning taste, Characteristic pleasant odor
• Antiseptic
• Preservative
• Mild counterirritant
• Rubbing alcohol is used as an
ü Astringent
ü Rubefacient
ü Mild local anesthetic
Isopropyl Alcohol
• Suitable substitute for ethanol
• But must not be ingested
• Primarily as a disinfectant for the skin and for surgical instruments
• Bactericidal in the concentration range of 50% to 95%
• A 40% concentration is considered equal in antiseptic efficacy to a 60% ethanol in water solution
Formaldehyde Solution
• Formalin is a colorless aqueous solution
• Used as a disinfectant for surface sterilization
• Contains not less than 37% w/v of formaldehyde (HCHO) with methanol added to retard polymerization
• The germicidal action of formaldehyde is slow but powerful
• The mechanism of action: denaturation of proteins
ü Direct nonspecific alkylation of nucleophilic functional group (amino, hydroxyl, and sulfhydryl) in proteins and nucleic acids to form carbinol derivatives
• Gutaraldehyde used to sterilize surgical instruments and surfaces contaminated with hepatitis virus
Phenol
• Phenol (carbolic acid) is a colorless to pale-pink crystalline material with a characteristic “medicinal odor
• Liquified phenol is simply phenol containing 10% water
• Bacteriostatic conc- 0.5%, Bactericidal-1%, fungicidal 1.5%
• MOA: denaturation of proteins
• Phenols & derivatives:-
p-Chlorophenol
• p-Chlorophenol is used in combination with camphor in liquid petrolatum as an external antiseptic and anti-irritant
Hexachlorophene
• Hexachlorophene, 2,2-methylene bis (3,4,6-trichlorophenol)
• 2,2- dihydroxy-3,5,6,3,5, 6-hexachlorodiphenylmethane
• Hexachlorophene is easily adsorbed onto the skin and Enters the sebaceous glands
• Topical application elicits a prolonged antiseptic effect
• Hexachlorophene is used in concentrations of 2% to 3% in
• Soaps
• Detergent creams
• Lotions
• Shampoos
• For various antiseptic uses
• Effective against gram-positive bacteria
• Many gram-negative bacteria are resistant
Resorcinol
• m- Dihydroxybenzene (resorcin)
• Resorcinol is only a weak antisepticit is used in 1% to 3% solutions
• Ointments and pastes in concentrations of 10% to 20% for the treatment of skin conditions
ü Ringworm
ü Eczema
ü Psoriasis
ü Seborrheic
ü Dermatitis
• Keratolytic agent
Hexylresorcinol
• 4-hexylresorcinol
• Effective antiseptic
• Bactericidal and fungicidal properties
Oxidizing agents:-
• Oxidizing agents that are of any value as germicidal agents depend on their ability to liberate oxygen in the tissues
• MOA: oxidation of sulphydryl groups of bacterial enzymes
• Hydrogen peroxide - H2O2
• Disinfectant and sterilant
• Benzoyl peroxide is both keratolytic and keratogenic
• It is used in the treatment of acne- exfoliant ,sebostatic
• Benzoyl peroxide induces proliferation of epithelial cell leading to sloughing and repair
Halogen – containing compounds:
Iodine tincture
• It is usually 2–7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water.
• It contains iodine which is an antiseptic. It is for use on minor wounds, cuts and scrapes.
Povidone–Iodine
• Charge-transfer complex of iodine with the nonionic surfactant PVP (poly vinyl pyrrolidine)
• Povidone–iodine is used as an aqueous solution for pre-surgical disinfection of the incision site
• Treating infected wounds and damage to the skin
• It is effective for local bacterial and fungal infections
Chlorine- Containing compounds
• All the chlorophores act by releasing chlorine which oxidezes the sulphydryl groups of bacterial enzymes and deactivates certain bacterial enzymes
• These compounds release hypochlorous acid when dissolved in water in the presence of acid – i.e HOCl is the active germicidal species.
• HOCl generates nascent oxygen to destroy the vital cellular machinery of microorganism
Chlorhexidine
• Chlorhexidine, also known as chlorhexidine gluconate (CHG), is a disinfectant and antiseptic that is used for skin disinfection before surgery and to sterilize surgical instruments-used both to disinfect skin of the patient and the hands of the healthcare providers.
• It is a germicidal mouthwash that reduces bacteria in the mouth. Chlorhexidine gluconate oral rinse is used to treat gingivitis (swelling, redness, bleeding gums).
Halazone
• P-dichlorosulfamoylbenzoic acid
• Faint chlorine odor
• The sodium salt of halazone is used to disinfect drinking water
Cationic surfactants:-
All cationic surfactants are quaternary ammonium compounds. They are always ionized in water and exhibit surface active properties.
They form micelles by concentrating at the interface of immiscible solvents-cationic head group has a high affinity for water and long hydrocarbon tail has an affinity for lipids and nonpolar solvents
• Benzalkonium chloride [Alkyl Benzyl dimethyl ammonium chloride is a mixture of alky benzyl dimethyl ammonium chloride]
• Benzalkonium chloride is a detergent, an emulsifier and a wetting agent
• It is used as an antiseptic for skin and mucous membranes
• Cetyl pyridinium chloride
• It is used as a general antiseptic for intact skin
• Irrigation of mucous membranes
Dyes
Gentian violet[Hexamethyl-p-rosaniline chloride](crystal violet,methyl violet)
• Antiseptic dye used to treat fungal infections of the skin (e.g., ringworm, athlete's foot).
• Also has weak antibacterial effects and may be used on minor cuts and scrapes to prevent infection.
Methylene blue[3,7-Bis(dimethylamino)-Phenazathonium chloride
• Weak antiseptic properties(bacteriostatic)
• Treatment of cystitis & urethritis
Nitrogen Compound:
Nitrofuran derivatives
• Nitrofurazone: [5-Nitro-2-furfuraldehyde semicarbazone]
• Furazolidone {3-[(5-nitrofurylidene) amino]-2-oxazolidinone}
• Has bactericidal action against a relatively broad range of intestinal pathogens including S.aureus, E.coli, Salmonella, Shigella, Proteus, Enterobacter and Vibriocholerae.
• Also active against the protozoan Giardia Lamblia.
• Used orally in the treatment of bacterial or protozoal diarrhea caused by susceptible organisms.
Mercury compounds:
Mercurials
• From early days, mercurial were used to treat skin infections & syphilis
• MOA: reversible nature of sulfhydryl group blockage
• Nitromersol [3-(hydroxy mercuri)-4-nitro-o-cresol]
• Thimerosal {sodium [(o-carboxy phenyl) thio] ethyl mercury}
Mode of Action
Alcohols and related compounds:-
• Ability to denature proteins & inhibit phosphorylation systems
• As the primary alcohol chain length increases, vanderwaal’s interactions increase and the ability to penetrate microbial membranes increases.
• As water solubility decreases the apparent anti-microbial potency diminishes. Branching of the alcohol chain decreases anti-bacterial potency. Weaker vanderwall’s forces brought about by branching do not penetrate bacterial cell membranes as efficiently. Yet, 2-propanol is used commercially instead of n-propyl alcohol as it is less expensive.
• The germicidal action of formaldehyde is slow but powerful. It is the direct, non-specific alkylation of nucleophilic functional groups (amino, hydroxyl, sulphydryl) in proteins and nucleophilic acids to form ‘Carbinol’ derivatives.
Phenols and Derivatives:-
• Phenols “denatures bacterial proteins at low concentrations”, “lysis of bacterial cell membranes at higher concentrations”
Oxidizing agents:-
• Germicidal action is based on their ability to liberate oxygen in the tissues.
• All these react in the tissues to generate oxygen and oxygen radicals. Oxidizing agents are especially effective against anaerobic bacteria and can be used in cleansing contaminated wounds.
Halogen-containing compounds:-
• Iodine acts to inactivate proteins by iodination of aromatic residues (Phenyl alanyl and tyrosyl) and oxidation (sulphhydryl groups).
• Chlorine released from compounds (Halozone) act by chlorination of amide nitrogen atoms &oxidation of sulfhydryl groups in proteins
Cationic Surfactants:-
• The mechanism of action involves the dissolution of the surfactant into the microbial cell membrane, destabilization and subsequent lysis.
• The surfactants may also interfere with enzymes associated with the cell membrane.
Dyes:-
• Acts on cell membranes. The difference in the susceptibility is related to the cellular characteristics. The cationic dyes are active against gram +ve bacteria and many fungi; gram –ve bacteria are generally resistant.
Nitrofurans:-
• The mechanism of action of Nitrofurans are not fully understood.
• These Nitrofurans are known to be mutagenic and carcinogenic under certain conditions. The cellular effects may be due to DNA damage caused by metabolic reaction products.
Preservatives
Ø “Preservatives are added to various dosage forms and cosmetic preparations to prevent microbial contamination”
Ø In parenteral and ophthalmic preparations, preservatives are used to maintain sterility in the event of accidental contaminations during use.
Ø Ideal preservative should be
• Effective at low concentrations against all possible microorganisms
• Non-toxic
• Compatible with other constituents used in the preparation
• Stable for the shelf life of the preparation.
Ideal preservative does not exist – most preservatives have some ideal features
• The preservatives are of 2 types
Ø Para-hydroxy benzoic acid derivatives
Ø Miscellaneous.
p-hydroxybenzoic acid derivatives (Parabens):-
• Esters of p-hydroxy benzoic acid have anti-fungal properties. Their toxicity to human host is generally low due to rapid hydrolysis to p-hydroxy benzoic acid, which is rapidly conjugated and excreted.
• Used as preservatives for liquid dosage forms
• Preservative effect increase with increase in molecular weight, but the methyl ester is more effective against ‘Molds’, where as the propyl ester is more effective against ‘Yeasts’.
• Eg, Methyl paraben – Methyl-p-hydroxybenzoate.
Others are propyl paraben (Propyl-p-hydroxybenzoate), Ethyl paraben (Ethyl-p-hydroxybenzoate) and Butyl paraben (Butyl-p-hydroxybenzoate)
Other preservatives:-
• Chlorobutanol: - 1,1,1-Trichloro-2-Methyl-2-Propanol
• Used as bacteriostatic in injections, ophthalmics & intranasal preparations
• Sodium benzoate-preservative in acidic liquid preparations
• Phenyl carbinol- used in ointments & lotions as an antiseptic in the treatment of pruritic infections
• Sodium propionate, Benzyl alcohol, Phenyl ethyl alcohol (2-Phenyl ethanol), Benzoic acid, Phenyl mercuric nitrate, Phenyl mercuric acetate, etc

























0 Comments: