
β-lactam Antibiotics - Medicinal Chemistry III B. Pharma 6th Semester
β-lactam Antibiotics
Contents
• β-lactam antibiotics – penicillins – History
• Structure and properties of penicillin
• Structure and activity relationships of penicillins
• Sensitivity of penicillins – due to acid, β-lactamase
• Narrow spectrum of activity of penicillins
• Extended (broad) spectrum Penicillins
• Chemical degradation of penicillins – various pathways
• Study of individual penicillins including structures and specific uses
• β-lactamase inhibitors – classification
• Mode of action of β-lactamase inhibitors
• Study of individual compounds used as β-lactamase inhibitors
Learning Objectives
At the end of this lecture, student will be able to
Discuss the structure and properties of Penicillins
Explain the SAR of penicillins
Explain the sensitivity of penicillins towards acid and enzymes
Describe the causes for narrow spectrum of activity of Penicillins
Discuss the structural modifications to design extended spectrum of penicillins
Discuss the chemical degradation of Penicillin
Discuss the structures, specific uses and side effects of penicillins
Classify β-lactamase inhibitors
Explain the mode of action of β-lactamase inhibitors
Discuss the structures, specific uses β-lactamase inhibitors
β-lactam Antibiotics
• Broad spectrum of antibacterial action. The unequaled importance of β-lactam antibiotics in chemotherapy is due to
- Potent lethal bactericidal action in the growth phase
- Low frequency of toxic and other adverse effects
MOA
• The lethal antibacterial action is due to the selective imbibition of bacterial cell wall synthesis. Specifically it inhibits the biosynthesis of peptidoglycan which provides strength and rigidity to the cell wall.
• Pencillins and cephalosporins acylate specific bacterial transpeptidases (Penicillin binding proteins) and make them inactive
• PBP 1a &1b – transpeptidases involved in peptidoglycansynthesis associated with cell elongation. Inhibition causes lysis
• PBP 2- transpeptidase involved in maintaining the rod shape in bacilli. Inhibition causes ovoid or round forms which undergo lysis
• PBP 3- transpeptidase required for septum formation in cell division. Inhibition results in formation of filamentous forms thast cannot separate
• PBP 4- carboxypeptidase responsible for the hydrolysis of the terminal peptide bonds of crosslinking peptides. This cleavage of bond is required before peptide crosslinkage. But inhibition of theses enzymes are not lethal
• The various β-lactam antibiotics differ in their affinities for the PBPs
β-lactam Antibiotics – Pencillins
• Structure of Penicillin was established in 1945 by “ DOROTHY HODGKINS” by the use of X-ray
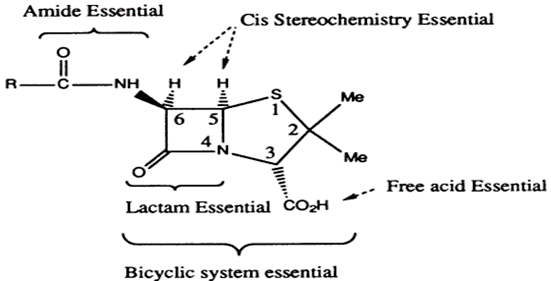
• Penicillin contains a bicyclic system consisting of a four-membered β-lactam ring fused to a five membered thiazolidine ring
• The skeleton of the molecule suggests that it is derived from the amino acids ‘CYSTEINE’ and ‘VALINE’
• The over-all shape of the molecule is like a HALF OPEN BOOK.
• It is a product of metabolism
• The acyl side chain (R) varies depending on the make-up of the fermentation media.
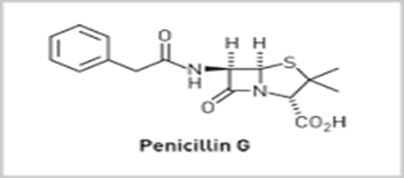
• For example, Corn steep liquor as the medium give penicillin G (R= Benzyl). This was due to the high levels of Phenyl acetic acid (PhCH2COOH) present in the medium.
Penicillin Analogues:-
• One method of varying the side chain is to add different carboxylic acids to the fermentation medium.
• For example, Addition of phenoxy acetic acid (PhOCH2COOH) gives Penicillin V.
Limitations:-
• Only acids of general formula ‘RCH2COOH’ can be added to the fermentation medium, which restricts the variety of analogues.
• Other disadvantages - Tedious -Time-consuming process.
Properties of Penicillin G:-
• Active against Gram +ve bacilli (Staphylococci, meningitis and gonorrhea) and many (but not all) Gram –ve Cocci.
• Non-toxic – The penicillins are amongst the safest drugs known to medicine.
• Not active over a wide range (or spectrum) of bacteria.
• Ineffective when taken orally since it breaks down in the acid condition of the stomach
• Penicillin G can only be administered by injection.
• Sensitive to all known β-lactamases. These are enzymes produced by penicillin-resistant bacteria which catalyzes the degradation of penicillins.
• Allergic reactions are suffered by some individuals
There are several problems associated with the use of Penicillin G; most serious ones
• Acid-sensitivity
• Sensitivity to penicillinase
• A narrow spectrum of activity
• The purpose of making semi-synthetic penicillin analogues is to find compounds which do not suffer from these disadvantages.
• So the study of ‘SAR’ and finding out the features important to its activity is vital for making new effective analogues of ‘Penicillin G’.
STRUCTURE –ACTIVITY RELATIONSHIPS OF PENICILLINS
• Fused β-lactam and thiazolidine ring forming a bicyclic system (Penam). Bicyclic system confers strain on the β-lactam ring
• The free carboxylic acid group is essential.
• The strained β-lactam ring is essential (increase strain, increase in activity, increase instability)
• The acyl amino side chain is essential except for thienamycin
Thienamycin
• Sulfur is usual but not essential
• The stereochemistry of the bicyclic ring with respect to the acyl amino side chain is important -Cis stereochemistry for the hydrogens
• Very little variation is tolerated by penicillin nucleus. Also, any variation which can be made is restricted to the acyl amino side chain
Acid sensitivity of Penicillins:-
There are THREE reasons for the acid sensitivity of ‘Penicillin G’
1) Ring Strain: The bicyclic system in penicillin consists of a four-membered ring and a five-membered ring. As a result, Penicillin suffers ‘LARGE ANGLE STRAIN and TORSIONAL STRAIN”.
• Acid catalyzed ring openings relieves these strains by breaking open the more highly stained four-membered lactam ring.
2) A highly reactive β-lactam carbonyl group:
• The carbonyl group in the β-lactam ring is highly susceptible to nucleophiles and does not behave like a normal tertiary amide which is usually quite resistant to Nucleophilic attack.
• This difference in reactivity is due mainly to the fact that stabilization of the carbonyl is possible in the tertiary amide, but is not possible in the β-lactam ring.
• The β-lactam nitrogen is unable to feed its lone pair of electrons into the carbonyl group since this would require the bi-cyclic rings to adopt an impossibly strained flat system.
• As a result the lone pair of electrons is localized on the nitrogen and the carbonyl group is far more electrophilic than one would expect for a tertiary amide.
• The normal tertiary amide is far less susceptible to nucleophiles since the resonance structures reduce the electrophilic character of the carbonyl group as follows:
3) Influence of the acyl side chain [Neighboring group participation]
• The neighboring acyl group can actively participate in a mechanism to open up the lactam ring. Thus penicillin G has self – destructive mechanism built into its structure
Tackling the problems of acid sensitivity:-
• Nothing can be done about the first two factors since the β-lactam ring is vital for antibacterial activity.
• Therefore, only the third factor can be tackled i.e. reducing the effect or participation of the neighboring group, by introducing a good electron withdrawing group attached to the carbonyl group. This electron-withdrawing group will draw the electrons away from the carbonyl oxygen by inductive effect and reduce its tendency to act as a nucleophile
• Influence of the acyl side chain [Neighboring group participation]
• For eg., Penicillin V, has an electronegative oxygen on the acyl side chain, so the molecule has better acid stability than Penicillin G and is stable enough to survive the acid in the stomach. Thus, it can be given orally
• However, Penicillin V is still sensitive to penicillinases and is slightly less active than penicilln G
Other eg.,
AMPICILLIN
OXACILLIN
Penicillin sensitivity to β-lactamases:
• β-lactamases are enzymes produced by penicillin-resistant bacteria, which can catalyse the reaction, in which the same ring opening and deactivation of penicillin which occurred with acid hydrolysis.
β-lactamases deactivation of penicillin
• The design of penicillinase-resistant penicillins involves the blocking of the penicillin from reaching the penicillinase active site.
• One way to do this is to “place a bulky group on the side chain”. Thus bulky group act as a ‘shield’ to prevent binding with the enzyme, penicillinase”.
• But if the shield is too bulky, then the steric shield also prevents the penicillin from attacking the enzyme responsible for bacterial cell wall synthesis.
• So the Ideal ‘Shield’ will be that which would be large enough to ward off the lactamase enzyme, but would be small enough to allow the penicillin to act on the enzyme responsible for cell wall synthesis.
• For eg. Methicillin was the first semi-synthetic penicillin unaffected by penicillinase and was developed to treat S.aureus infections, which was due to virulent penicillin – resistant strains. Both the methoxy groups (ortho) on the aromatic ring are important in shielding the lactam ring.
• ‘Methicillin’ is not an ideal drug, since there are no electron-withdrawing groups on the side chain, it is acid sensitive, and so has to be injected.
• It has ⅕ of the activity of Penicillin G against organisms sensitive to Penicillin G, it shows poor activity against some streptococci, and it is inactive against Gram –ve bacteria. This problem of acid sensitivity was solved by incorporating into the side chain a five-membered heterocycle which was designated to act as a steric shield and also to be electron-withdrawing.
• These compounds (Oxacillin, Cloxacillin and Flucloxacillin) are resistant to acid and penicillinase and are useful against S. aureus infections.
• The only difference between the above three compounds is the type of halogen substitution on the aromatic ring.
• The influence of these groups is found to be pharmacokinetic i.e. they influence such factors as absorption of the drug and plasma protein binding.
• For eg. Cloxacillin is better absorbed through the gut wall than Oxacillin, whereas flucloxacillin is less bound to plasma protein, resulting in higher levels of the free drug in the blood.
• These also are inactive against Gram –ve bacteria.
• Hence, acid resistant penicillin would be the choice of the drug against an infection.
• However, if the bacteria proved resistance because of penicillinase enzyme, then the therapy would be changed to penicillinase – resistance penicillin.
Narrow – spectrum of activity:-
• Most penicillins show a poor activity against Gram –ve bacteria.
There are several reasons for this resistance.
Permeability barrier:-
• It is difficult for penicillins to invade a Gram –ve bacterial cell because of the make of the cell wall.
• Gram –ve bacteria have a coating on the outside of their cell wall which consists of a mixture of fats, sugars and proteins.
• This coating can act as barrier in various ways.
• For eg. The outer surface may have an overall –ve and +ve charge depending on its constituent triglycerides.
• An excess of phosphatidyl Glycerol would result in an overall anionic charge whereas an excess of lysyl phosphatidyl Glycerol would result in an overall cationic charge.
• Penicillin has a free carboxylic acid which if ionized, would be repelled by the former type of cell coat.
• Alternatively, the fatty portion of the coating may act as a barrier to the polar hydrophilic penicillin molecule.
• The only way in which penicillin can pass such a barrier is through protein channels in the outer coating-but most of these are usually closed.
• High levels of transpeptidase enzyme produced:-
• The transpeptidase enzyme is the enzyme attacked by penicillin. In some Gram –ve bacteria, a lot of transpeptidase enzyme is produced and the penicillin is incapable of inactivitating all the enzyme molecules present.
Modifications of the transpeptidase enzyme:-
• A mutation may occur which allows the bacterium to produce a transpeptidase enzyme which is not antagonized by penicillin.
Presence of β-lactamase:-
• β-lactamases are enzymes which degrade penicillin. They are situated between the cell wall and its outer coating.
Transfer of the β-lactamase enzyme:-
• Bacteria can transfer small portion of DNA from one cell to another through structures called Plasmids. These are small pieces of circular bacterial DNA. If the transferred DNA contains the code for the β-lactamase enzyme, then the recipient cell acquires immunity.
Extended (Broad) Spectrum Penicillins
• In order to tackle the problem of narrow spectrum activity all the above factors has to be considered. But the changes are confined only to the variations in the side chain.
• Introduction of hydrophilic groups on the side chain (eg., Penicillin G) favour activity against Gram +ve bacteria, but has poor activity against Gram –ve bacteria.
• If hydrophilic groups on the side chain have either little effect (eg., Penicillin T) or cause a reduction of Gram +ve activity (eg., Penicillin N). But they lead to an increase in activity against Gram –ve bacteria.
• Enhancement of Gram –ve activity is found to be greatest if the hydrophilic groups (e.g., NH2, OH, COOH) is attached to the carbon that is ‘Alpha’ to the carbonyl group on the side chain.
• Pencillins which are active against, both Gram +ve and Gram –ve bacteria are known as broad-spectrum antibiotics.
There are two classes of broad-spectrum antibiotics
• Both classes have an ‘alpha’ hydrophilic group
• Class I broad spectrum antibiotics: Ampicillin and Amoxycillin
• *where hydrophilic group is –NH2 group:
• Class II broad spectrum antibiotics: Carbenicillin
• *where hydrophilic group is acid group,
Chemical Degradation of Penicillins:-
• The main cause of deterioration of penicillins is the reactivity of the strained β-lactam ring, particularly by hydrolysis.
• The course of the hydrolysis and the nature of the degradation products are influenced by the pH of the solution.
• The β-lactam carbonyl group of penicillin readily undergoes Nucleophilic attack by water or hydroxide ion to form inactive ‘Penicilloic acid’ which is reasonably stable in neutral to alkaline solutions but readily undergoes de-carboxylation and further hydrolytic reactions in acidic solutions.
• Other Nucleophiles as hydroxyl amines, alkyl amines and alcohols – open the β-lactam ring to form the corresponding hydroxamic acids, amides and esters.
• In strongly acidic solution (pH < 3), penicillin undergoes a complex series of reactions forming a variety of inactive degradation products. The first step in the rearrangement to the penicillanic acid. This process is initiated by protonation of the β-lactam nitrogen, followed by Nucleophilic attack of the acyl oxygen atom on the β-lactam carbonyl carbon.
• Subsequent opening of the β-lactam ring destabilizes the thiazolidine ring, suffers acid-catalyzed ring opening to form penicillanic acid.
• Penicillanic acid is very unstable and undergoes TWO major degradation pathways.
• ‘I Path’ is hydrolysis of oxozolone ring to form unstable penamaldic acid, an enamine easily undergoes hydrolysis to penicillamine (a major degradation product) and penaldic acid.
• ‘II Path’ involves a complex rearrangement of penicillanic acid to penillic acid.
• Penillic acid (an imidazoline -2-carboxylic acid) readily decarboxylates and on hydrolytic ring opening to form major end product penilloic acid.
• Penicilloic acid (cannot be detected as intermediate), the major product formed. Weakly acidic (neutral?) to alkaline hydrolytic conditions (also enzymatic conditions), exists in equilibrium with penamaldic acid, undergoes decarboxylation to give penilloic acid.
• The third major product of the degradation is penicilloaldehyde formed by decarboxylation of penaldic acid (a derivative of malonaldehyde) .
Individual Compounds:
Penicillin G: [Benzyl penicillin]
• Agent of choice for the treatment of different kinds of bacterial infections than any other antibiotic.
• Inactive orally. But by combining antacids as calcium carbonate, aluminium hydroxide and magnesium trisilicate or a strong buffer as sodium citrate and by giving large doses, as it is poorly absorbed from intestinal tract, will increase the oral activity of the drug.
• Water soluble potassium or sodium salts are used orally and parenterally to achieve high plasma concentration of pencillin G rapidly.
All pencillins should be administered with caution due to allergic side effects
Penicillin V [Phenoxy methyl Penicillin]
• It is resistant to hydrolysis by gastric acid and it produces uniform concentration in blood, (when administered orally).
• For parenteral solution, potassium salt is usually used.
Cloxacillin:[3-(o-chlorophenyl)-5-methyl-4-isoxasolyl] Penicillin sodium.
• Oxacillin, cloxacillin and dicloxacillin are highly resistant to inactivation by penicillinase. The steric effects of 3-phenyl and 5-phenyl groups prevent binding to the B- lactamase active site.
• Prescence of Cl in ortho position causes increase in activity due to increase in oral absorption. It attains high plasma levels
Naficillin sodium
• [6-(2-ethoxy-1-naphthyl) penicillin sodium]
• Ethoxy group and second ring of naphthalene group increase the stability against penicillinase.
• Stable to acid so it can be given by oral route
• Used in infection caused solely by penicillin G-resistant staphylococci or streptococci.
• Also effective against pneumococci & group a – β-hemolytic streptococci.
• Should be administered with care, due to its allergic side effects.
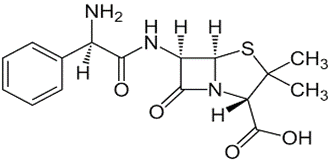
Ampicillin: 6-[D-α-aminophenyl acetamido] Penicillanic acid or D-α-amino benzyl penicillin
• It has an anti-bacterial spectrum broader than that of Penicillin G.
• Active against Gram –Ve organism that are susceptible to other penicillins
• Acid resistant
• More active against Gram –ve bacteria and enterococci than other penicillins
• Not resistant to penicillinases
• Use:- Particularly useful for the treatment of acute urinary tract infections caused by E.Coli or Proteus mirailis.
• It is the agent of choice against Haemophilic influenzae infections
• Used in combination with Probenicid for the treatment of Gonorrhea.
• Effective in treating Salmonellosis and Shigellosis.
Amoxicillin: 6-[D-α-amino-p-hydroxy phenyl acetamido] Penicillanic acid (semi synthetic penicillin)
• Its antibacterial spectrum is same as that of ampicillin (resistant to acid, susceptible to alkaline and β-lactamase hydrolysis).
• More complete gastro-intestinal absorption to give higher plasma and urine levels.
• Less diarrhea.
• Little or no effect of food on absorption.
β-lactamase inhibitors (Suicidal substrates)
• The β-lactamase inhibitors, such as sulbactam and tazobactam and natural occurring β-lactams, such as the thienamycins, inhibit both β-lactamases and interact with penicillin binding protein (PBP) present in the bacterial cell wall.
• β-lactamase inhibitors are given along with β-lactamase sensitive penicillin, so that they competitively bind to the enzyme and protect the penicillin from destruction.
• β-lactamase inhibitors are of 2 classes.
• Class. I – inhibitors:- have a heteroatom at position 1
• Eg. Clavulanic acid & sulbactam.
Clavulanic acid
Sulbactam
• Class. II – inhibitors:- do not have a heteroatom at position 1
Eg. Carbapenams – as Thienamycin
Mode of action of β-lactamase inhibitors:-
• Inactivation of β-lactamases is done by mechanism-based inhibitors, which act by reacting with the enzyme in the same way as that of the substrate.
• An acyl enzyme intermediate is formed by the reaction of the β-lactam with an active-site serine hydroxyl group of the enzyme.
• For normal substrates (Penicillins), the acyl enzyme intermediate readily undergoes hydrolysis, destroying the substrate and freeing the enzyme to attack more substrate.
• For mechanism based-inhibitor, the acyl enzyme intermediate formed is diverted by tautomerism to a more stable imine that undergo hydrolysis more slowly to eventually free the enzyme (transient inhibitors).
• Because these inhibitors are also substrates for the enzymes that they inactivate, they are sometimes called as ‘Suicidal substrates’
Mechanism based inhibition of β-lactamase
Individual Compounds of β-lactamase inhibitor:-
Clavulanate potassium:-
• Clavulanic acid is isolated from Streptomyces Clavuligeris
• *it is 1-oxapenam
• *lacks 6-acylamino side chain
• *but possess 2-hydroxyethylidene moiety at C-2.
• It has very weak anti-bacterial activity
• It is a potent inhibitor of S.aureus β-lactamase and plasmid-mediated β-lactamases produced by Gram-ve bacilli.
• Combination with Amoxacillin and potassium salt of Clavulanic acid (Augmentin) is intended for the treatment of skin, respiratory, ear and urinary tract infections caused by β-lactamase producing bacterial strains- the oral bioavailability of both are the same
• It is effective against strains which are resistant to Amoxacillin alone
• It is stable to acid
• Combination of potassium clavulanate and ticarcillin sodium (extended–spectrum penicillin) is recommended for Septicemia, lower respiratory tract infections and urinary tract infections, bone and joint infections, skin & structure infections caused by β-lactamase producing strains of S.aureus, Klebsiella, E.coli, P.aeruginosa and other pseudomonas spp. Citrobacter spp. Enterobacter spp. Serratia Marcescens, etc
Ticarcillin disodium:-
Ticarcillin disodium ‘α-carboxy-3-thienyl penicillin’
Class II inhibitors – Carbepenams
• Class of highly effective antibiotic agents commonly used for the treatment of severe or high-risk bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections.
• Broadest coverage of antibacterial activity
• Including Gm+, and Gm- (especially drug resistant species), anaerobic coverage -Cover MSSA, Enterococcus, streptococcus spp.
• Drugs of choice for ESBL infections (ESBL-producing bacteria can't be killed by many of the antibiotics )
Carbapenams:
Thienamycin
• First isolated from of Streptomyces Cattleya.
• ONLY two structural features of thienamycins are shared with the penicillins and cephalosporins.
• A fused bicyclic ring system containing a β-lactam.
• An equivalently attached 3-carboxyl group.
• The presence of double bond between C-2 and C-3 in the bicyclic structure creates a considerable ring strain and increases the reactivity of the β-lactam to ring opening reactions.
• It has a 1-hydroxylethyl gp at 6th position (not the acyl amino side chain) & this is oriented to the α ring
• It has broad spectrum antibacterial properties-active against most Gram+ & gram- bacteria and resistant to activation by most β-lactamases (could be because of hydroxyl ethyl side chain)
• It is more susceptible to acid & alkaline hydrolysis coz the strain nature of the fused ring system. Stable at pH between 6-7
Imipenam
• Very stable to most β-lactamases. It is an inhibitor of β-lactamases from certain Gram+ve & Gram-ve bacteria resistant to other β-lactamam antibiotics
• It is used for the treatment of a wide variety of bacterial infections of the skin & tissues, lower respiratory tract, bones & joints and genitourinary tract infections.
• They are also used for septicemia & endocarditis caused by β-lactamases producing strains





































0 Comments: