
Partition coefficient - Medicinal Chemistry
PARTITION COEFFICIENT
Contents
• Introduction
• Physico chemical properties
• Partition coefficient
Intended learning outcomes
At the end of this lecture, student will be able to:
• Explain the importance of partition coefficient in biological action steps to calculate the partition coefficient
INTRODUCTION
Drug molecules interact with biological structures
â
Drug effect
Lipoproteins/enzymes membranes
Nucleic acids
Drug effect is preceded by drug transport from site of application to site of action Is dependent on physiochemical properties.
PHYSICOCHEMICAL PROPERTIES
Interatomic distances
Intermolecular forces
Stereochemistry
Partition coefficient
Solubility
Ionization
All affect pharmacokinetics
PHARMACOKINETICS
PARTITION COEFFICIENT
• Hydrophobic bonding interactions are critical
• It can be approximated by partition coefficient
• Useful to know the hydrophobic bonding properties of substituent groups.
• Hydrophobic bonding constant π for a substituent is obtained as a difference In log P.
π = log PX - log PH
PX = PC for substituted compound
Thus, π describes the substituent.
REPRESENTATIVE π VALUES
| Substituent | Aromatic | Aliphatic |
| C6H11 | 2.51 | 2.51 |
| n-C4H9 | 2.00 | 2.00 |
| Cl | 0.76 | 0.39 |
| H | 0.00 | 0.00 |
| NO2 | -0.28 | -0.82 |
| COOH | -0.28 | -1.26 |
| OH | -0.67 | -1.16 |
Calculation steps of Log P for OMA
(i) The molecule is dissected into its various groups, functionalities and substitutents.
(ii) Appropriate hydrophilic/lipophilic fragment constants are assigned and summed
(iii) Compounds with log Pcalc values greater than +0.5 are considered water insoluble (lipophilic) and those with log Pcalc values less than +0.5 are considered water soluble (hydrophilic).
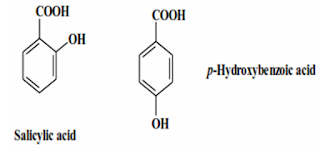
Calculated log P Values for salicylic acid and p-Hydroxybenzoic acid:
| Salicylic acid | p-Hydroxybenzoic acid | ||
| Value | Fragment | Value | Fragment |
| Phenyl | +2.0 | Phenyl | +2.0 |
| OH | -1.0 | OH | -1.0 |
| COOH | -0.7 | COOH | -0.7 |
| IMHB | +0.65 | - | - |
| Sum | +0.95 | | +0.3 |
| Prediction | Water insoluble | Prediction | Water soluble |
Lipophilicity
Lipophilicity (‘fat-liking’) is the most important physical property of a drug in relation to its absorption, distribution, potency, and elimination.
Lipophilicity is often an important factor in all of the following, which include both biological and physicochemical properties:
• Solubility
• Absorption
• Plasma protein binding
• Metabolic clearance
• Volume of distribution
• Enzyme / receptor binding
• Biliary and renal clearance
• CNS penetration
• Storage in tissues
• Bioavailability
• Toxicity
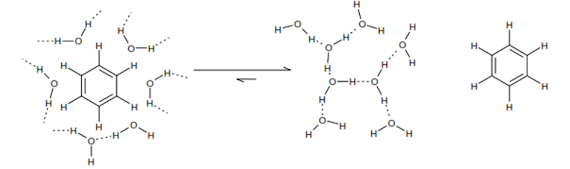
The hydrophobic effect
Molecular interactions – why don’t oil and water mix?
• This is entropy driven (remember δG = δH – TδS). Hydrophobic molecules are encouraged to associate with each other in water.
• Placing a non-polar surface into water disturbs network of water-water hydrogen bonds. This causes a reorientation of the network of hydrogen bonds to give fewer, but stronger, water-water H-bonds close to the non- polar surface.
• Water molecules close to a non-polar surface consequently exhibit much greater orientational ordering and hence lower entropy than bulk water.
This principle also applies to the physical properties of drug molecules.
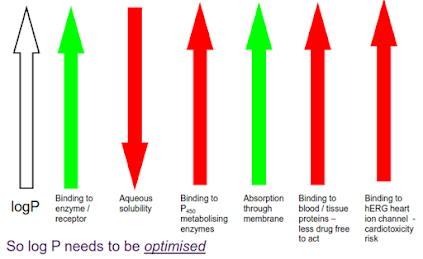
If a compound is too lipophilic, it may
• be insoluble in aqueous media (e.g. gastrointestinal fluid or blood)
• Bind too strongly to plasma proteins and therefore the free blood concentration will be too low to produce the desired effect
• distribute into lipid bilayers and be unable to reach the inside of the cell
Conversely, if the compound is too polar, it may not be absorbed through the gut wall due to lack of membrane solubility.
So it is important that the lipophilicity of a potential drug molecule is correct. How can we measure this?
Partition coefficients
Partition coefficient P (usually expressed as log10P or logP) is defined as:
P =[X]octanol / [X]aqueous
P is a measure of the relative affinity of a molecule for the lipid and aqueous phases in the absence of ionisation.
1-Octanol is the most frequently used lipid phase in pharmaceutical research. This is because:
• It has a polar and non-polar region (like a membrane phospholipid)
• Po/w is fairly easy to measure
• Po/w often correlates well with many biological properties
• It can be predicted fairly accurately using computational models
Calculation of logP
LogP for a molecule can be calculated from a sum of fragmental or atom-based terms plus various corrections.
logP = ∑ fragments + ∑ corrections
clogP for windows output
C: 3.16 M: 3.16 PHENYLBUTAZONE
| Class | Type | Log(P) Contribution Description | Value |
| FRAGMENT | # 1 | 3,5-pyrazolidinedione | -3.240 |
| ISOLATING | CARBON | 5 Aliphatic isolating carbon(s) | 0.975 |
| ISOLATING | CARBON | 12 Aromatic isolating carbon(s) | 1.560 |
| EXFRAGMENT | BRANCH | 1 chain and 0 cluster branch(es) | -0.130 |
| EXFRAGMENT | HYDROG | 20 H(s) on isolating carbons | 4.540 |
| EXFRAGMENT | BONDS | 3 chain and 2 alicyclic (net) | -0.540 |
| RESULT | 2.11 | All fragments measured | clogP 3.165 |
What else does logP affect?
Distribution coefficients
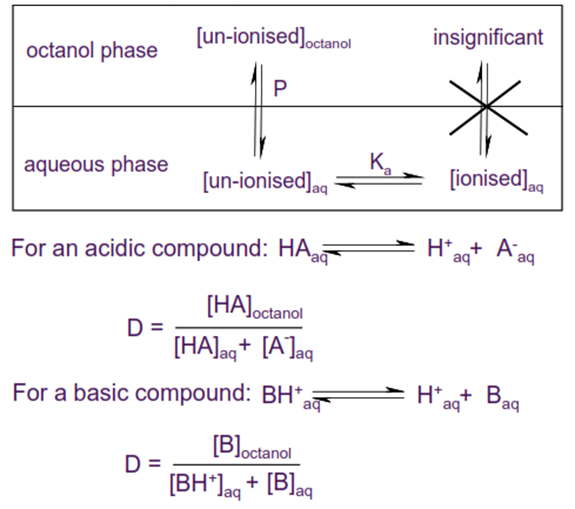
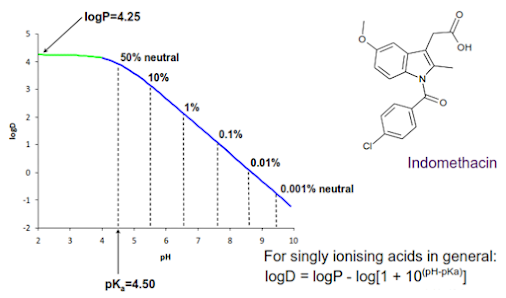
• If a compound can ionise then the observed partitioning between water and octanol will be pH dependent.
• Distribution coefficient D (usually expressed as logD) is the effective lipophilicity of a compound at a given pH, and is a function of both the lipophilicity of the un-ionised compound and the degree of ionisation.
Relationship between logD, logP and pH for an acidic drug
pH - Distribution behaviour of bases
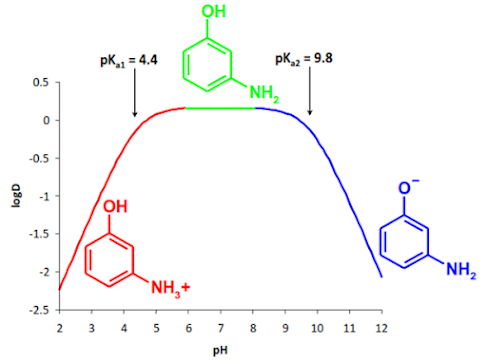
pH - Distribution behaviour of amphoteric compounds
How can lipophilicity be altered?
SUMMARY
• Drug effect is preceded by drug transport from site of application to site of action Is dependent on physiochemical properties.
• Hydrophobic bonding constant Useful to know the hydrophobic bonding properties of substituent groups












0 Comments: