
IONISATION - Medicinal Chemistry
IONISATION
Contents
• Introduction
• Ionisation
• Effect of ionisation on antibacterial potency of sulphonamides
• Effect of substituents on ionisation
Intended learning outcomes
At the end of this lecture, student will be able to:
• Explain the importance of ionisation of drug molecules on biological action
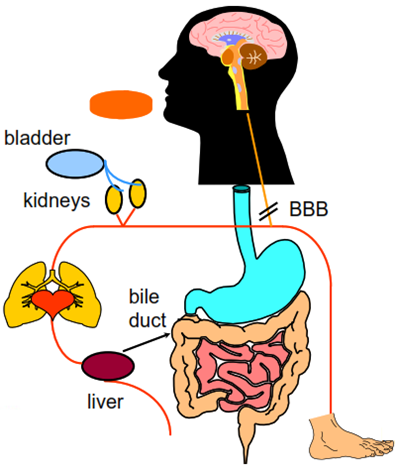
What must a drug do other than bind?
An oral drug must be able to:
• dissolve
• survive a range of pHs (1.5 to 8.0)
• survive intestinal bacteria
• cross membranes kidneys
• survive liver metabolism
• avoid active transport to bile
• avoid excretion by kidneys
• Partition into target organ
• avoid partition into undesired places (e.g. brain, foetus)
Why are physical properties important in medicinal chemistry?
• So, before the drug reaches its active site, there are many hurdles to overcome.
• Many complicated biological processes can be modelled using simple physical chemistry models or properties and understanding these often drives both the lead optimisation and lead identification phases of a drug discovery program forward.
• This lecture will focus on oral therapy, but remember that there are lots of other methods of administration e.g. intravenous, inhalation, topical. These will have some of the same, and some different, hurdles.
Reducing the complexity
| Biological process in drug action | Underlying physical chemistry | Physical chemistry model |
| Dissolution of drug in gastrointestinal fluids | Energy of dissolution; lipophilicity & crystal packing | Solubility in buffer, acid or base |
| Absorption from small intestine | Diffusion rate, membrane partition coefficient | logP, logD, polar surface area, hydrogen bond counts, MWt |
| Blood protein binding | Binding affinity to blood proteins e.g. albumin | Plasma protein binding, logP and logD |
| Distribution of compound in tissues | Binding affinity to cellular membranes | logP, acid or base |
Ionisation
• Ionisation is protonation or deprotonation resulting in charged molecules
• About 85% of marketed drugs contain functional groups that are ionised to some extent at physiological pH (pH 1.5 – 8).
The acidity or basicity of a compound plays a major role in controlling:
• Absorption and transport to site of action - Solubility, bioavailability, absorption and cell penetration, plasma binding, volume of distribution
• Binding of a compound at its site of action - un-ionised form involved in hydrogen bonding
• Elimination of compound - Biliary and renal excretion, CYP P450 metabolism
How does pH vary in the body?
| Fluid | pH | Fluid | pH |
| Aqueous humour | 7.2 | Saliva | 6.4 |
| Blood | 7.4 | Small intestine | 6.5 |
| Colon | 5-8 | Stomach (fasting) | 1.4-2.1 |
| Duodenum (fasting) | 4.4-6.6 | Stomach (fed) | 3-7 |
| Duodenum (fed) | 5.2-6.2 | Sweat | 5.4 |
| Urine | 5.5-7.0 | | |
• So the same compound will be ionised to different extents in different parts of the body.
• This means that, for example, basic compounds will not be so well absorbed in the stomach than acidic compounds since it is generally the unionised form of the drug which diffuses into the blood stream.
Ionisation constants
• The equilibrium between un-ionised and ionised forms is defined by the acidity constant Ka or pKa = -log10 Ka
• For an acid:
• For a base:
When an acid or base is 50% ionised: pH=pKa
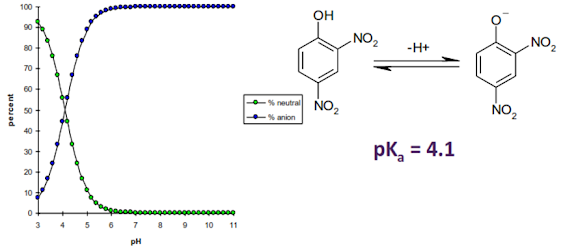
Ionisation of an acid – 2, 4-dinitrophenol
Ionisation of an base – 4-aminopyridine
Effect of ionisation on antibacterial potency of sulphonamides
• From pH 11 to 7 potency increases since active species is the anion.
• From pH 7 to 3 potency decreases since only the neutral form of the compound can transport into the cell.
SUMMARY
• Ionisation is protonation or deprotonation resulting in charged molecules
• About 85% of marketed drugs contain functional groups that are ionised to some extent at physiological pH (pH 1.5 – 8).
• Basic compounds will not be so well absorbed in the stomach than acidic compounds since it is generally the unionised form of the drug which diffuses into the blood stream.





0 Comments: