
Anthelmintics - Medicinal Chemistry III B. Pharma 6th Semester
Anthelmintics
• Anthelmintics are drugs that have the capability of ridding the body of parasitic worms or helminths
• Helminths that infect human hosts are divided into two categories, or phyla:
• a) Platyhelminthes (flatworms)- include the classes Cestode (tapeworms) and Trematode (flukes or schistosomes)
• b) Aschelminthes or nematodes (roundworms)- roundworm, hookworm, pinworm, and whipworm. These worms are cylindrical in shape, with significant variations in size, proportion, and structure
• Nematode Infections
• Ancylostomiasis or Hookworm Infection- American hookworm (Necator americanus) and the “Old World” hookworm (Ancylostoma doudenale).
• Enterobiasis or Pinworm Infection- Enterobius vermicularis
• Ascariasis or Roundworm Infections- Ascaris lumbricoides
• Trichuriasis or Whipworm Infections- Trichuris trichiura
• Trichinosis or Trichina Infection- Trichinella spiralis
• Filariasis- Wuchereria bancrofti, Brugia malayi, and Brugia timori
• Cestode and Trematode Infections
• Cysticercosis or Tapeworm Infection
• Beef tapeworm (Taenia saginata)
• Pork tapeworm (Taenia solium)
• Dwarf tapeworm (Hymenolepis nana)
• Fish tapeworm (Diphyllobothrium latum)
• Schistosomiasis or Blood Flukes- Schistosoma hematobium, Schistosoma mansoni, and Schistosoma japonicum
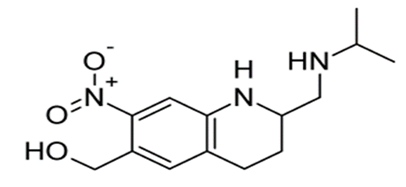
Diethylcarbamazine citrate
• Highly water-soluble crystalline compound that has selective anthelmintic activity
• It is effective against various forms of filariasis, including Bancroft, onchocerciasis, and laviasis
• It is also active against ascariasis
• Mechanim- not clearly known
• Suggestion- inhibition of microtubule polymerization and disruption of preformed microtubules
• Or interference with arachidonic acid metabolism
• Adverse reactions- anaphylactic reactions, intense pruritus, and ocular complications
Thiabendazole
• Occurs as a white crystalline substance that is only slightly soluble in water but is soluble in strong mineral acids.
• Thiabendazole is a basic compound with a pKa of 4.7 that forms complexes with metal ions
• Mechanism- inhibits the helminth-specific enzyme fumarate reductase (important enzyme in helminthes that appears to be involved in oxidation of NADH to NAD for ATP production)
• Also arrest nematode cell division in metaphase by interfering with microtubule assembly and exhibit a high affinity for tubulin, the precursor protein for microtubule synthesis
• Has broad-spectrum anthelmintic activity
• It is used to treat enterobiasis, strongyloidiasis (threadworm infection), ascariasis, uncinariasis (hookworm infection), and trichuriasis (whipworm infection)
• Also used to relieve symptoms associated with cutaneous larva migrans (creeping eruption) and the invasive phase of trichinosis.
• Widely used in veterinary practice to control intestinal helminths in livestock
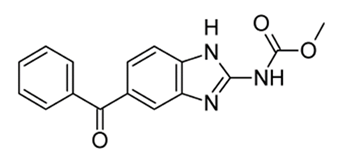
Mebendazole
• Broad-spectrum anthelmintic that is effective against various nematode infestations, including whipworm, pinworm, roundworm, and hookworm
• Mechanism- irreversibly blocks glucose uptake in susceptible helminths, thereby depleting glycogen stored in the parasite
• It apparently does not affect glucose metabolism in the host. It also inhibits cell division in nematodes
• Poorly absorbed by the oral route
• Adverse reactions are uncommon and usually abdominal discomfort
• It is teratogenic in laboratory animals and should not be given during pregnancy
Albendazole
• Broad-spectrum anthelmintic that is not currently marketed in North America
• Widely used throughout the world for the treatment of intestinal nematode infection
• It is effective as a single-dose treatment for ascariasis, New and Old World hookworm infections, and trichuriasis
• Multiple-dose therapy with albendazole can eradicate pinworm, threadworm, capillariasis, clonorchiasis, and hydatid disease
• Effectiveness of albendazole against tapeworms (cestodes) is generally more variable and less impressive
• White crystalline powder that is virtually insoluble in water
• Oral absorption of albendazole is enhanced by a fatty meal
• Drug undergoes rapid and extensive first-pass metabolism to the sulfoxide, which is the active form in plasma
• Elimination half-life of the sulfoxide ranges from 10 to 15 hours
• High dose can result in adverse effects such as bone marrow depression, elevation of hepatic enzymes, and alopecia
Niclosamide
• Occurs as a yellowish white, water-insoluble powder
• Potent taeniacide that causes rapid disintegration of worm segments and the scolex
• Penetration of the drug into various cestodes appears to be facilitated by the digestive juices of the host
• Niclosamide is well tolerated following oral administration, and little or no systemic absorption of it occurs
• A saline purge 1 to 2 hours after ingestion of the taeniacide is recommended to remove the damaged scolex and worm segments- mandatory
Oxamniquine
• Antischistosomal agent that is indicated for the treatment of Schistosoma mansoni (intestinal schistosomiasis) infection
• Mechanism- Shown to inhibit DNA, RNA, and protein synthesis in schistosomes
• 6-hydroxymethyl group is critical for activity;
• metabolic activation of precursor 6-methyl derivatives is critical
• Free base occurs as a yellow crystalline solid that is slightly soluble in water but soluble in dilute aqueous mineral acids and soluble in most organic solvents
• Dizziness and drowsiness are common, but transitory, side effects
• Serious reactions, such as epileptiform convulsions, are rare
Praziquantel
• Broad-spectrum agent that is effective against various trematodes (flukes)
• It has become the agent of choice for the treatment of infections caused by schistosomes (blood flukes)
• Effective treatment for fasciolopsiasis (intestinal fluke), clonorchiasis (Chinese liver fluke), fascioliasis (sheep liver fluke), opisthorchosis (liver fluke), and paragonimiasis (lung fluke)
• Mechanism- increases cell membrane permeability of susceptible worms, resulting in the loss of extracellular calcium. Massive contractions and ultimate paralysis of the fluke musculature occurs, followed by phagocytosis of the parasite
• Oral administration, about 80% of the dose is absorbed
• Drug is rapidly metabolized in the liver in the first-pass
• White crystalline solid that is insoluble in water
Ivermectin
• Is a mixture of 22,23-dihydro derivatives of avermectins B1a and B1bprepared by catalytic hydrogenation
• Avermectins are members of a family of structurally complex antibiotics produced by fermentation with a strain of Streptomyces avermitilis
• Ivermectin is active in low dosage against a wide variety of nematodes and arthropods that parasitize animals
• Structure- pentacyclic 16-membered–ring aglycones glycosidically linked at the 3-position to a disaccharide that comprises two oleandrose sugar residues
• Side chain at the 25-position of the aglycone is sec-butyl in avermectin B1a, whereas in avermectin B1b, it is isopropyl
avermectins B1a(-C2H5) and B1b (-CH3)
• Ivermectin contains at least 80% of 22,23-dihydroavermectin B1a and no more than 20% 22,23-dihydroavermectin B1b
• Widespread use in veterinary practice in the United States and many countries throughout the world for the control of endoparasites and ectoparasites in domestic animals
• It has been found effective for the treatment of onchocerciasis (“river blindness”) in humans, an important disease caused by the roundworm Oncocerca volvulus
• Mechanism- It blocks interneuron–motor neuron transmission in nematodes by stimulating the release of the inhibitory neurotransmitter GABA
Diethylcarbamazine citrate- Synthesis
Mebendazole- Synthesis









0 Comments: