
NSAIDs- Non Steroidal Anti-inflammatory Drugs - Medicinal Chemistry
NSAIDs- Non Steroidal Anti-inflammatory Drugs
INTENDED LEARNING OUTCOMES
At the end of the lecture, students will be able to
• Define NSAIDs.
• Understand the general mechanism of action of NSAIDs
• Categorize the NSAIDs according to their chemical structure
• Outline the synthesis of some NSAIDs
• Recognize the specific uses of the different NSAIDs
Contents
• NSAIDs-Non Steroidal Anti-inflammatory Drugs
• The general mechanism of action of NSAIDs
• NSAIDs classification according to their chemical structure
• The synthesis of some NSAIDs
• The specific uses of the different NSAIDs
NSAIDS
• NSAIDS are the drugs which are used against Inflammation by inhibiting the COX Enzyme.
Mechanism of Action of NSAIDS
Classification
1. SALICYCLIC ACID DERIVATIVES
• ASPIRIN
• DIFLUNISAL
• SALSALATE
• SULPHASALAZINE
2. P-AMINO PHENOL DERVIVATIVES
• PARACETAMOL
• PHENACETIN
3. PYRAZOLIDINE DIONE DERIVATIVES
• PHENYL BUTAZONE
• OXYPHENBUTAZONE
• SULPHIN-PYRAZONE
4. ANTHRANILIC ACID DERIVATIVES
• MEFENEMIC ACID
• FLUFENEMIC ACID
• MECLOFENAMATE
5. ARYL ALKANOIC ACID DERIVATIVE
A. INDOLE ACETIC ACID: INDOMETHACIN
B. INDENE ACETIC ACID: SULINDAC
C. PYRROLE ACETIC ACID: TOLMETIN, ZORMIPIRAC
D. PHENYL ACETIC (PROPIONIC) ACID:
• IBUPROFEN, DICLOFENAC,
• NAPROXEN, CAPROFEN,
• FENOPROFEN, KETO-PROFEN,
• FLURBIPROFEN, KETOROLAC, ETODAOLAC
6. OXICAMS
• PIROXICAM
• MELOXICAM
• TENOXICAM
7. SELECTIVE COX-2 INHIBITORS
• CELECOXIB
• ROFECOXIB
• VALDECOXIB
8. GOLD COMPOUNDS
•AURONOFIN
•AUROTHIOGLUCOSE
•AUROTHIOGLUCAMIDE
• AUROTHIOMALATE SODIUM
9. MISCELLANEOUS
•NABUMETONE
•NIMESULIDE
•ANALGIN
10. DRUG USED IN GOUT
•ALLOPURINOLL
• PROBENECID
• SULPHINPYRAZONE
I. Salicylates
• Salicylates not only posses antipyretic, analgesic, and anti-inflammatory properties
• But also other actions that have been proven to be therapeutically beneficial because salicylates promote the excretion of uricacid
• They are useful in the treatment of gouty arthritis
• More attention has been given to the ability of salicylates (aspirin) to inhibit platelet aggregation, which may contribute to heart attack and strokes, and
• Hence, aspirin reduces the risk of myocardial infarction
• In addition, a recent study suggested that aspirin and other NSAIDs might be protective against colon cancer
ASPIRIN:
Chemical Formula:C9H8O4
Chemical structure of Aspirin
Properties:Aspirin is a white crystalline powder, slightly soluble in water and soluble in alcohol
Mode of action of Aspirin
Aspirin inhibits the enzyme cycloxygenase and thromboxane synthetase (TxA2) by binding irreversibly and interfering with the platelet aggregation
Medicinal Uses:
• Relief of minor aches and mild-to-moderate pain in the conditions such as arthritis and related arthritic condition --- Anti Rheumatic
• Also used in myocardial infarction prophylaxis
• Analgesic
• Anti-pyretic
Dosage forms:
• Aspirin tablets I.P., B.P.,
• Dispersible aspirin tablets B.P.,
• Effervescent soluble aspirin tablets B.P.,
• Gastro-resistant aspirin tablets B.P.,
• Aspirin and Caffeine tablets B.P.,
• Co-codaprin tablets B.P.,
• Dispersible co-codaprin tablets B.P.
SODIUM SALICYLATE:
Chemical Name:Sodium Salicylate, Monosodium salicylate; Benzoic acid, 2-hydroxy-, monosodium salt; B.P., U.S.P., Eur. P., Int. P., Ind. P.,
Chemical Structure of Sodium Salicylate:
Properties:Sodium salicylate is a white crystalline powder, soluble in water, sparingly soluble in alcohol.
Medicinal Uses:
• It is used for fever and for the relief of pain
• It also possesses anti-inflammatory actions similar to aspirin
• Symptomatic therapy of gout
II. p-Amino phenol derivatives
• These derivatives possess analgesic and antipyretic action, but lack anti-inflammatory effects
• Acetanilide was introduced into the therapy in 1886 as an antipyretic–analgesic agent
• However, it was subsequently found to be too toxic, having been associated with methemaglobinemia and jaundice
• Phenacetin was introduced in the following year and was widely used but was withdrawn recently because of its nephrotoxicity
• Acetaminophen (paracetamol) was introduced in 1893 and it remains the only useful agent of this group used as an antipyretic and an analgesic agent
Paracetamol INN, BAN, Acetaminophen USAN
Chemical Name:4′-Hydroxyacetanilide; Acetamide, N-(4- hydroxyphenyl)-
Chemical Structure of Paracetamol
Properties:Paracetamol exist as white crystalline powder, sparingly soluble in water, soluble in alcohol, and very slightly soluble in methylene chloride
Mechanism of action of Paracetamol:
• Paracetamol produce antipyresis by acting on the hypothalamic heat-regulating centre and analgesia by elevating the pain threshold NHCOCH3
Paracetamol Toxicity:
•Hepatic necrosis and death have been observed following over dosage hepatic damage is likely in an adult who takes more than 10 g in a single dose or if a 2-year old child takes more than 3 g
Medicinal Uses:
•It is a metabolite of acetanilide and phenacetin employed as an antipyretic and analgesic
•It may be used effectively in a broad spectrum of arthritic and rheumatic conditions linked with musculoskeletal pain, headache, neuralgias, myalgias, and dysmenorrhea.
•It is particularly useful in aspirin-sensitive patients.
Paracetamol Dosage forms:
• Paracetamol tablets I.P, B.P.,
• Paracetamol syrup I.P., Co-codamol tablets B.P.,
• Effervescent Co-codamol tablets B.P.,
• Co-dydramol tablets B.P., Co-proxamol tablets B.P.,
• Paracetamol capsules B.P.,
• Paediatric paracetamol oral solution B.P.,
• Paracetamol oral suspension B.P.,
• Paracetamol suppositoriesB.P.,
• Dispersible paracetamol tablets B.P.,
• Soluble paracetamol tablets B.P.
Phenacetin INN, BAN, USAN
Chemical structure of Phenacetin
Chemical Name: p-Acetophenetidide; Acetamide, N-(4- ethoxyphenol) - ; Acetophenetidin; p-Ethoxyacetanilid; B.P. (1973), U.S.P., Eur. P., Int. P., Ind. P.
Properties: It exists as a white glistering powder with a bitter taste, sparingly soluble in water and soluble in chloroform.
Medicinal Uses:
• It is an analgesic and an antipyretic with similar effectiveness as an aspirin.
• It has a greater potential for toxicity (hemolytic anaemia and methemoglobinaemia) than paracetamol.
• Irreversible kidney damage with prolonged ingestion of phenacetin has been established which ultimately resulted in complete withdrawal of this drug in many countries.
Pyrazolidine dione derivatives
3, 5-Pyrazolidinediones
Phenyl butazone:It is a pyrazole derivative
Phenylbutazone INN, BAN, USAN
Chemical Name:4-Butyl-1, 2-diphenyl-3, 5-pyrazolidinedione; 3, 5- Pyrazolidinedione, 4-butyl-1, 2-diphenyl- ;Butadione ; B.P., U.S.P., Eur. P., Int. P.,
Chemical structure of Phenyl butazone
Properties: Phenylbutazone is a white crystalline powder, practically insoluble in water, sparingly soluble in alcohol, and soluble in alkaline solutions.,
Medicinal Uses:
• Antipyretic analgesic, and anti-inflammatory actions, because of its toxicity it is not used as a general antipyretic or analgesic.
• It is a usual practice reserved for use in the treatment of osteoarthrosis, ankylosing spondylitis, arthritis, acute superficial thrombophlebitis, painful shoulder, and Reiter’s disease, where less toxic drugs have failed.
Phenazone INN, BAN, Antipyrine USAN (Antipyrine)
Chemical structure of Phenazone
Chemical Name: 2,3-Dimethyl-1-phenyl-3-pyrazolin-5-one; 1,2- Dihydro-1,5-dimethyl-2-phenyl-3H-pyrazol-3- one; Antipyrine; Phenazone B.P., Eur. P., Int. P., Antipyrine U.S.P.
Medicinal Uses:
• As antipyretic, it possesses local anaesthetic and styptic actions and solutions containing 5% are used locally as ear drops.
• It has now been replaced by relatively more effective and safer drugs.
IV. Anthranilic acid derivatives
Mefenamic Acid BAN
Chemical structure of Mefenamic acid
Chemical Name: N-(2, 3-Xylyl) anthranilic acid; Benzoic acid, 2-[(2, 3-dimethylphenyl) amino] - ; B.P.,
Medicinal Uses:analgesic and anti-infl ammatory agent
Synthesis of Mefenamic acid:
• It may be prepared by the condensation of o-chlorobenzoic acid with 2, 3-xylidine in the presence of potassium carbonate to give the potassium salt of mefenamic acid, which on treatment with hydrochloric acid yields the official compound mefenamic acid
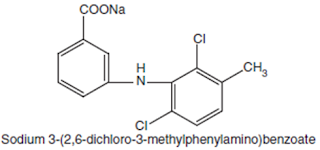
Meclofenamate Sodium BAN, USAN, Meclofenamic Acid INN.
Chemical structure of Meclofenamate Sodium
Chemical Name: Monosodium N-(2, 6-dichloro-m-tolyl) anthranilate monohydrate; Benzoic acid, 2-[2, 6-(dichloro- 3-methylphenyl) amino]-, monosodium salt; U.S.P.,
Medicinal Uses:It possesses analgesic, anti-inflammatory, and antipyretic properties. It is used for the treatment of acute and chronic rheumatoid arthritis and osteoarthritis.
V. Arylalkanoic acids
a. Indole acetic acid derivatives
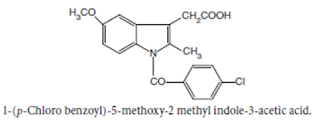
Indomethacin BAN, USAN, Indomethacin INN
Chemical structure of Indomethacin
Chemical Name:1-(p-Chlorobenzoyl)-5-methoxy-2- methylindole-3-acetic acid ; 1H-Indole-3-acetic acid, 1-(4- chlorobenzoyl)-5-methoxy-2-methyl- ; BP ; USP
Properties: It is a white or yellow crystalline powder, insoluble in water and sparingly soluble in alcohol.
• Indomethacin is more effective than aspirin
Medicinal Uses:
• It is a non-steroid drug possessing anti-inflammatory, antipyretic and analgestic properties.
• It is usually used for the treatment of rheumatoid arthritis, ankylosing (rheumatoid) spondylitis, gouty arthritis and osteoarthritis.
• It is not an ordinary simple analgesic and owing to its reasonably serious untoward effects should be used with great caution
• The most frequent side effects are gastric distress and headache
b. Indene acetic acid derivatives
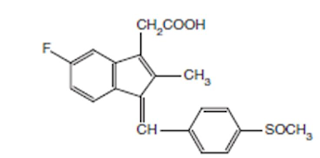
Sulindac INN, BAN, USAN
Chemical structure of Sulindac
Chemical Name:cis-5-Fluoro-2-methyl-1-[(p-methylsulfinyl) benzylidene] indene-3-acetic acid ; 1H-Indene-3-acetic acid, 5-fluoro-2-methyl-1-[[4- (methylsulfinyl) phenyl] methylene]-, (Z)-; USP
• It is a fluorinated indene with a structural resemblance to indomethacin
Properties:Suindac is a yellow crystalline powder, very slightly soluble in water, soluble in methylenechloride, and dilute solutions of alkali hydroxides, sparingly soluble in alcohol
Medicinal Uses:
• It is usually employed in the treatment of rheumatic and musculoskeletal disorders;
• For severe and long-term relief of signs and symptoms of acute painful shoulder, acute gouty arthritis and osteoarthritis
c. Pyrrole acetic acid derivative
Tolmetin Sodium BAN, USAN, Tolmetin INN
Chemical structure of Tolmetin sodium
Chemical Name:Sodium 1-methyl-5-p-toluoylpyrrole-2-acetate dihydrate; 1H-Pyrrole-2-acetic acid, 1-methyl-5-(4- methylbenzoyl)-, sodium salt, dihydrate; USP
Properties: It is a light yellow, crystalline powder, soluble in water, slightly soluble in alcohol.
Medicinal Uses:
• As an antipyretic, analgesic, and anti-infl ammatory actions.
• It is employed in the treatment of rheumatic and musculoskeletal disorders.
Zomepirac Sodium BAN, USAN, Zomepirac INN
Chemical Structure of Zomepirac Sodium
Chemical Name:Sodium 5-(p-chlorobenzoyl)-1, 4-dimethylpyrrole-2- acetate dihydrate ; 1H-Pyrrole-2-acetic acid, 5-(4- chlorobenzoyl)-1, 4- dimethyl-, sodium salt, dihydrate ; USP ;
• It is an analgesic and anti-inflammatory drug structurally very similar to tolmetin sodium.
• It is normally used in mild to moderate pain, including that of musculoskeletal disorders.
d. Phenyl acetic (propionic) acid:
Diclofenac Sodium BAN, USAN, Diclofenac INN
Chemical Structure of Diclofenac sodium
Chemical Name: Sodium [o-(2, 6-dichloroanilino) phenyl] acetate; Benzene-acetic acid, 2-[(2, 6-dichlorophenyl) amino]-, monosodium salt; Dichlorophenac sodium
Properties:Diclofenac sodium is a white or slightly yellowish crystalline slightly hygroscopic powder, sparingly soluble in water, soluble in methanol and alcohol, slightly soluble in acetone.
Medicinal Uses:in the treatment of rheumatic arthritis.
Ketorolac
Chemical Name: 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid
Chemical Structure of Ketorolac
Properties:Colourless compound freely soluble in water
• A pyrrolizine carboxylic acid derivative structurally related to INDOMETHACIN.
• It is an NSAID Ketorolac non-selective inhibits the enzymes cyclooxygenase 1 (COX-1) and COX-2
Medicinal Uses:
• Used principally for its analgesic activity
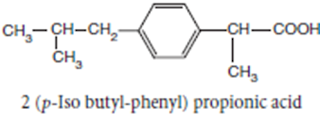
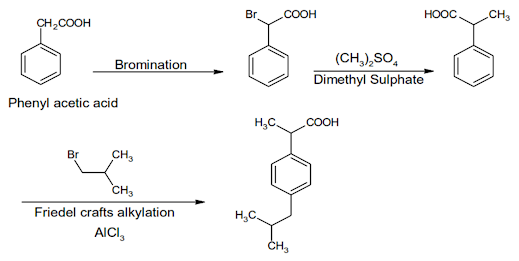
Ibuprofen INN, BAN, USAN
Chemical Name: -p-Isobutylhydratropic acid; Benzeneacetic acid, α- methyl-4-(2-methyl-propyl)
Chemical Structure of Ibuprofen
Properties:Ibuprofen is a white crystalline powder or colourless crystals, practically insoluble in water, soluble in acetone, methanol, methylene chloride, and dilute solutions of alkali hydroxides and carbonates
• Ibuprofen is a propionic acid derivate and nonsteroidal anti- inflammatory drug (NSAID). Ibuprofen inhibits the activity of cyclo- oxygenase I and II
Medicinal uses:
• Anti-inflammatory
• Analgesic
• Antipyretic effects
Ibuprofen Synthesis:
Naproxen INN, BAN, USAN
Chemical Structure of Naproxen
Chemical Name:2-(6-Methoxy-2-naphthyl)-propionic acid; (+)-6- Methoxy-α-methyl-2-naphthaleneacetic acid ; 2-Naphthaleneacetic acid, 6-methoxy-α-methyl-, (+)- ; BP ; USP
Properties:Naproxen is a white crystalline powder, practically insoluble in water, soluble in ethanol and in methanol.
Medicinal Uses:
• It possesses analgesic, anti-inflammatory, and antipyretic actions
• It is used in the treatment of rheumatic arthritis, dysmenorrhea, and acute gout
VI. OXICAMS
Piroxicam INN, BAN, USAN,
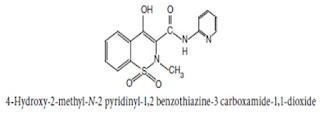
Chemical Structure of Piroxicam
Chemical Name:4-Hydroxy-2-methyl-N-2-pyridyl-2H-1, 2- benzothiazine-3-carboxamide 1, 1-dioxide ; 2H-1, 2- benzothiazine-3- carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1, 1-dioxide
Properties and uses:Piroxicam is a white or slightly yellow crystalline powder, practically insoluble in water, soluble in methylene chloride, and slightly soluble in ethanol.
Medicinal Uses:
• It is employed for acute and long-term therapy for the relief of symptoms of osteoarthritis and rheumatoid arthritis.
• It also possesses uricosuric action and has been used in the treatment of acute gout
SUMMARY
• NSAIDS are the drugs which are used against Inflammation by inhibiting the COX Enzyme
• Some NSAIDs are only used topically eg. Methyl salicylate, sodium salicylate
• Along with anti-inflammatory action, most NSAIDs also possess analgesic and anti-pyretic action.





















0 Comments: