
Drug classification and Solubility - Medicinal Chemistry
Solubility
Contents
• Drug classification
• Physio chemical properties influencing biological action
• Solubility
• Solubility prediction
Intended learning outcomes
At the end of this lecture, student will be able to:
• Explain the importance of solubility of drug molecules on biological action.
Drug Classification
Classified according to their origin:
Pure organic compounds are the chief source of agents for the cure, mitigation or the prevention of disease. These remedial agents could be classified according to their origin:
• Natural compounds:materials obtained from both plant and animal, e.g. vitamins, hormones, amino acids, antibiotics, alkaloids, glycosides…. etc.).
• Synthesis compounds: either pure synthesis or synthesis naturally occurring compounds (e.g. morphine, atropine, steroids and cocaine) to reduce their cost.
• Semi-synthesis compounds: Some compounds either cannot be purely synthesized or cannot be isolated from natural sources in low cost. Therefore, the natural intermediate of such drugs could be used for the synthesis of a desired product (e.g. semi synthetic penicillins).
Classified according to their Use:
Drugs can be classified according to their medicinal uses into two main classes:
I- Pharmacodynamic agents: Drugs that act on the various physiological functions of the body (e.g. general anaesthetic, hypnotic and sedatives, analgesic etc.).
II- Chemotherapeutic agents: Those drugs which are used to fight pathogenic (e.g. sulphonamides, antibiotics, antimalarial agents, antiviral, anticancer etc.).
Drugs can treat different types of diseases:
1- Infectious diseases: Born (transmitted) from person to person by outside agents, bacteria (pneumonia, salmonella), viruses (common cold, AIDS), fungi (thrush, athletes foot), parasites (malaria)
2- Non-infectious diseases: disorders of the human body caused by genetic malfunction, environmental factors, stress, old age etc. (e.g. diabetes, heart disease, cancer. Haemophilia, asthma, mental illness, stomach ulcers, arthritis).
3- Non-diseases:alleviation of pain (analgesic), prevention of pregnancy (contraception), anesthesia.
Physico-chemical properties in relation to biological action
• Drug action results from the interaction of drug molecules with either normal or abnormal physiological processes. Drugs normally interact with targets (which they are proteins, enzymes, cell lipids, or pieces of DNA or RNA).
• The ability of a chemical compound to elicit a pharmacologic /therapeutic effect is related to the influence of its various physical and chemical (physicochemical) properties
• The most pharmacologically influential physicochemical properties of organic medicinal agents (OMAs) are:
1. Solubility
2. Acidity and basicity
3. Reactivity
SOLUBILITY
• Drugs must be in solution to interact with receptors.
• Drugs have some degree of solubility in both aqueous and lipid compartments (PC).
• Solubility is a function of:
1- Ionization
2- Molecular structure
3- Molecular weight
4- Stereochemistry
5- Electronic structure
Importance of solubility:
(1) Formulation of the drug in an appropriate dosage form and
(2) Bio-disposition: Disposition of OMAs in the living system after administration (absorption, distribution, metabolism, and excretion).
• The solubility expression: in terms of its affinity/philicity or repulsion/phobicity for either an aqueous (hydro) or lipid (lipo) solvent.
hydrophilic....................water loving
lipophobic.....................lipid hating
lipophilic.......................lipid loving
hydrophobic..................water hating
• Drug administered orally as a solid or in suspension have to dissolve in the aqueous gastric fluid (dissolution)Before they can be absorbed and transported via systemic circulation to their site of action
• The rate & extent of dissolution of a drug is a major factor in controlling the absorption of that drug.
• This is because the concentration of the drug in fluid in gut lumen is one of the main factors governing the transfer of the drug through the membranes
SOLUBILITY OF ORGANIC MEDICINAL AGENTS
• Majority of OMAs possess balanced solubility (have some degree of solubility in both aqueous and lipid media).
• Because there is a need for OMAs to move through both aqueous (plasma, extracellular fluid, cytoplasm, etc.) and lipid media (biologic membranes) in the biological system.
• Solubility of OMAs should be viewed as being on a continuum between high lipophilicity on one end of the spectrum and high hydrophilicity on the other.
• In order for a chemical compound to dissolve in a particular solvent/medium the compound must establish attractive forces between itself and molecules of the solvent.
• In order for a chemical compound to dissolve in a particular solvent/medium the compound must establish attractive forces between itself and molecules of the solvent.
• It is possible to estimate the solubility properties of an OMA (hydrophilic vs. lipophilic) by examining the structure of the OMA and noting whether its structural features promote affinity for aqueous or lipid media.
• The most important intermolecular attractive forces (bonds) that are involved in the solubilization process are:
1. Van der Waals Attraction
• Weakest intermolecular force (0.5-1.0 kcal/mole)
• Electrostatic
• Occurs between nonpolar groups (e.g. hydrocarbons)
• Highly distance and temperature dependent
2. Dipole-Dipole Bonding
• Stronger (1.0 to 10 kcal/mole)
• Occurs electrostatically between electron deficient and electron excessive /rich atoms (dipoles)
• Hydrogen bonding is a specific example of this bonding and serves as a prime contributor to hydrophilicity
3. Ionic Bonding
• Electrostatic attraction between cations and anions
• Common in inorganic compounds and salts of organic molecules
• Relatively strong (5
4. Ion-Dipole Bonding
• Electrostatic between a cation/anion and a dipole
• Relatively strong (1-5 kcal/mole)
• Low temperature and distance dependence
• Important attraction between OMAs and H2O
Solubility Prediction
• The relative solubility of an OMA is a function of the presence of both lipophilic and hydrophilic features within its structure, which serve to determine the extent of interaction of the OMA with lipid and/or aqueous phases.
• The relative solubility of an OMA can be determined in the laboratory, i.e. the partition coefficient [P; the ratio of the solubility of the compound in an organic solvent to the solubility of the same compound in an aqueous environment (i.e., P=[Drug]lipid/[Drug]aqueous). P is often expressed as a log value.
• A mathematical procedures also have been developed to estimate the relative solubility of an organic molecule based upon differential contributions of various structural features to overall solubility.
• For example, the relative solubility of an OMA is the sum of the contributions of each group and substituent to overall solubility.
Example:
Examination of the structure of chloramphenicol (indicates the presence of both lipophilic (nonpolar) and hydrophilic (polar) groups and substituents.
The presence of oxygen and nitrogen containing functional groups usually enhances water solubility. While lipid solubility is enhanced by non ionizable hydrocarbon chains and ring systems.
1. Laboratory Estimation of Relative Solubility
The relative solubility of an organic compound is measured by determining the extent of its distribution into an aqueous solvent (usually pH 7.4 buffer) and a lipid solvent (usually n-octanol). These experiments generate a value, P, the partition coefficient for that particular compound.
2- Mathematical Estimation of Relative Solubility
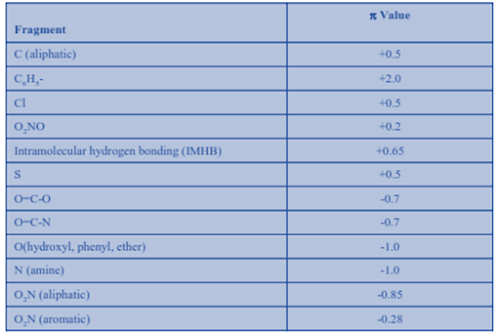
Solubility contributions (groups and substituents) are expressed as hydrophilic (negative value) or lipophilic (positive value) fragment constants.
Log Pcalc = ∑ π
Where; Log P calc = log of partition cofficient and ∑ π = sum of hydrophilic-lipophilic constants.
Hydrophilic-Lipophilic constants
SUMMARY
• Drug can be classified into Pharmacodynamic agents and chemotherapeutic agents
• The most pharmacologically influential physicochemical properties of organic medicinal agents (OMAs) are: solubility, acidity and basicity
• The relative solubility of an organic compound is measured by determining the extent of its distribution into an aqueous solvent (usually pH 7.4 buffer) and a lipid solvent (usually n-octanol).



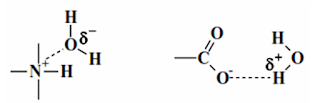







0 Comments: