
Cephalosporins & Monobactams - Medicinal Chemistry III B. Pharma 6th Semester
β-lactam Antibiotics
Cephalosporins & Monobactams
Contents
• Cephalosporins - Principal antibiotic components
• Nomenclature of Cephalosporins
• Structures of Cephalosporins
• Classification of Cephalosporins
• Study of individual compounds
• Monobactams – introduction
• SAR of monobactams
Learning Objectives
• At the end of this lecture, student will be able to
• Discuss Cephalosporins and their principal components
• Explain the nomenclature of Cephalosporins
• Describe the structure of Cephalosporin
• Classify Cephalosporins
• Discuss the SAR of Cephalosporins
CEPHALOSPORINS
Ø Cephalosporins are β-lactam antibiotics isolated from Cephalosporium spp., or prepared semi-synthetically.
Ø Three principal antibiotic components isolated from the fungus are
- Cephalosporin P1:- A steroid with minimal anti-bacterial activity
- Cephalosporin N:- Later identified as Synnematin N (a penicillin derivative called as Penicillin N)
- Cephalosporin C
Penicillin N: - [D-(5-Amino-5-Carboxy pentanoyl)-Penicillanic acid]
Cephalosporin C:- [3-Acetoxy-methyl-7-(5’-Amino-5’-Carboxy pentanoyl amino) Cephalosporanic acid]
• Cephalosporin is a close congener of penicillin N containing dihydrothiazine ring instead of thiazolidine ring, of the penicillin.
Nomenclature:-
• The fused ring system is designated as 5-thia-1-azabicyclo[4.2.0]Oct-2-ene.
• So in this system
• For example, Cephalothin is ‘3-(acetoxy methyl)-7-[2-(thienyl acetyl)amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]-Oct-2-ene-Carboxylic acid’.
• A simplified method is that the saturated bicyclic ring system with the lactam carboxyl oxygen is called as ‘Cepham’ (as penam for penicillins). According to this system all Cephalosporins and Cephamycis are named as 3-Cephams (or ∆3- Cephams) to designate the position of double bond.
• ‘Cepham’
• ‘Cephalosporanic acid’
Cephalosporins
Structure of Cephalosporins:-
• At – 7 position – Acyl group:- The effect of modifications acyl group on the activity of the cephalosporins is similar to that of penicillins
• At -3 position – Alkyl, Allylic group, acetoxy group:- provides a reactive site at which various 7-acyl amino Cephalosporinic acid structures can easily be varied by Nucleophilic substitution.
• Reduction of the ‘3-acetoxymethyl’ –to 3-methyl substitution to prepare 7-amino-desacetyl cephalosporanic acid (7-ACDA) derivatives can be done by catalytic hydrogenation.
v In the preparation of semi-synthetic cephalosporins following improvements are sought.
Ø Increased acid stability.
Ø Improved pharmacokinetic properties, particular better oral absorption.
Ø Broadened anti-microbial spectrum
Ø Increase activity against resistant microorganisms (i.e. increase in resistance to enzymatic destruction, increase in penetration and increase receptor affinity)
Ø Decreased allergenicity
Ø Increase in tolerance after parenteral administration.
CEPHALOSPORINS – Classification
Cephalosporins are divided into
First, Second, Third and Fourth – generation agents.
• This is based roughly on their time of discovery and their anti-microbial properties.
• Progression from Ist to IV generation is associated with broadening of the gram-ve antibacterial spectrum, some reduction in activity against gram +ve organism and enhanced resistance to β-lactamases
Ø I Generation (in the order)
• Cephalexin, Cephradine, Cefadroxil, Cephalothin, Cephapirin and Cefazolin.
Ø II Generation (in the order)
• Cefachlor, Loracarbef, Cefprozil, Cefamandole, Cefonicid, Ceforanide, Cefoxitin, Cefotetan, Cefmetazole, Cefuroxime, Cefdopoxime.
Ø III Generation (in the order)
• Cefixime, Cefoperazone, Cefotaxime, Ceftizoxime, Ceftriaxone, Ceftazidine, Ceftibuten.
Ø IV Generation (in the order)
• Cefepime, Cefpirome
Oral Cephalosporins
v Oral activity of cephalosporins is due to increased acid stability of β-lactam ring
Ø Phenylglycyl substituent confers this acid stability (at 7th position) due to the presence of protonated amino group on the 7-acylamino portion of the molecule.
Ø Another important factor for their excellent oral activity is due to the “carrier mediate transport of the dipeptide – like, Zwetterionic Cephalosporins” -“similar to α-aminobenzyl penicillin (Ampicillin)”
Ø The other important factor for high acid stability (good oral activity) of the Cephalosporins is the absence of a leaving group at the 3-position
Ø Cephalexin and cephachlor are orally active due to the prescence of phenylglycyl substitution at 7th position and absence of a good leaving group at 3rd position
• So, despite the presence of the phenylglycyl side chain in its structure Cephalosporanic acid derivative ‘Cephaloglycin’ is poorly absorbed orally because of hydrolysis of the 3-acetoxy group in the low pH of the stomach.
• The resulting 3-hydroxy derivative is poorly absorbed and it undergoes lactonization under acidic conditions.
• The hydroxyl derivatives and the lactones are very less active.
• Orally activity can also be conferred in certain Cephalosporins by esterification of the 4-carboxylic acid group to form acid stable , lipophilic esters that undergo hydrolysis in the plasma.
• Eg., Cefuroxime axetil
- Cefpodoxime proxetil
- These are β-lactamase – resistance alkoximino-cephalosporins
- They are orally active ester prodrug derivatives of Cefuroxime and Cefpodoxime respectively
CEPHALOSPORINS – Study of individual compounds
Cephalexin:- 7-(D-α-amino- α-phenyl-acetomido)-3-methyl-Cephem-4-Carboxylic acid
• It is ‘orally active’ as it is acid resistant
• Food does not interfere with its absorption
• Used for the treatment of urinary tract infection and upper respiratory tract infection
Cephradine:- [Partially hydrogenated derivative of Cephalexin]
• It is orally and parenterally active (only derivative)
• Has similar anti-bacterial and pharmacokinetic properties as Cephalexin
• Used for the treatment of uncomplicated urinary tract infections and upper respiratory tract infections.
Cefadroxil :- [7-{(D-p-hydroxyl phenyl glycyl) amino} -3- methyl-Cephem-4-carboxylic acid.
• It is ‘orally active’
• It has prolonged duration of action (slow urinary excretion)
• Anti-bacterial spectrum of action and therapeutic activity are very similar to Cephalexin and Cephradine.
Cefuroxime sodium: [2ndGeneration Cephalosporin]
• 7-{α-(methoxy imino)-α-(2-furyl)-acetylamino} – 3-{carbamoyl oxymethyl} – Cephem-4-carboxylic acid.
• It is active orally & parenterally
• ‘Syn alkoximino’ substituent is associated with β-lactamase activity.
• Active against β-lactamase –producing strains such as E.Coli, K. pneumonia, N-gonorrrhoeae and H. influenza.
• Effective in meningitis caused by susceptible organisms
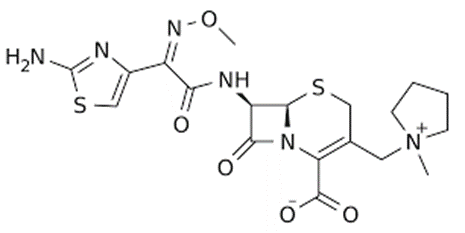
Cefixime:- [3rdgeneration Cephalosporin][7{(α-carboxy methyl oxyimino)-α-(2-amino-thiazol-4-yl) acetyl –amino} -3- vinyl –Cephem-4-Carboxylic acid
• It is ‘orally active’
• Broad spectrum activity-resistant to many β-lactamases
• treatment of a variety of respiratory tract infections- acute bronchitis, pharangitis, toslitis, etc
• Treatment of uncomplicated UTI & gonorrhea caused by β-lactamase producing strains
Cefepime
• β-Lactamase resistant
• Broad antibacterial spectrum
• Treatment of UTI, lower respiratory tract infections, skin & soft tissue infections, chronic osteomyelitis
MONOBACTAMS (Monolactams)
• Monobactams (Monolactams) are those antibiotics with isolated β-lactam ring.
• Sulfazecin is the prototype compound isolated from saprophytic soil bacteria. It has weak antibacterial properties, but highly resistant to β-lactamases.
The structure of ‘Sulfazecin’ is
Ø Other compound is ‘Aztreonam disodium’, has useful properties as an antibacterial agent
• The structure of ‘Aztreonam disodium’ is
• Aztreonam – prepared by total synthesis. It is used to treat urinary and lower respiratory tract infections, intra abdominal infections and gynecological infections as well as septicemias.
• The third compound is ‘Tigemonam’- which is a newer monobactam-is orally active
• Tigemonam’ is highly resistant to β-lactamases. Its antibacterial spectrum resembles that of Astreonam.
• Use: Used for oral treatment of urinary tract infections and other non-life threatening infections caused by β-lactamases producing Gram-ve bacteria
SAR of Monobactams:
• A 3-methoxy group – contributes to low antibacterial potency and poor chemical stability of these antibiotics.
• Eg., Sulfazecin
• A 4-methyl group – increases stability to β-lactamases and actitity against gram-ve bacteria but decrease actitity against gram+ve bacteria
• Eg., Aztreonam
• A 4,4-gem – dimethyl substitution slightly decreases antibacterial potency after oral administration.
• Eg., Tigemonam
• Presence of heterocyclic ring system on the acyl side chain increases the antibacterial activity and increased resistance against β-lactamases.




















0 Comments: