
Plant Tissue Culture – Requirements and Callus culture
Plant Tissue Culture – Requirements and Callus culture
Content
• General Introduction
• Types of Tissue Culture
• Plant Tissue Culture
• Callus Culture
Objective
At the end of the lecture the student will able to:
• Discuss the role of tissue culture in production of sec metabolites
• Discuss the technique of callus culture
• Explain the role of nutrients of tissue culture media
Explant Preparation
• Explant: Initial piece of the plant introduced in vitro or detached portion of plant body used in tissue culture
• Age of explant
• Optimum success – explants - healthy vigorous plants
• Meristems, shoot tip, auxillary bud and immature, rapidly growing tissues - must be selected as explants
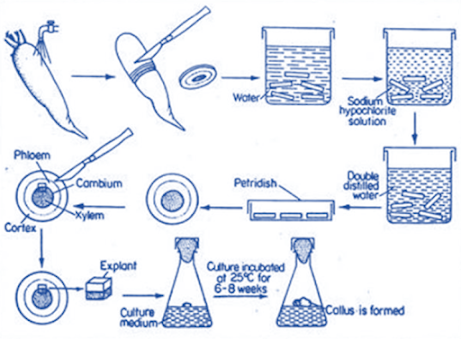
• Surface sterilization Disinfection – explant – surface microorganisms – presence of any contaminant – interfere with the growth of explant
Explant
• Spore dormant microorganisms – resistant - disinfectants – antibiotics incorporated – medium or explant is dipped
• Explants –priory washed with soap solution – dipped in ethanol – disinfectants solution for 10-20 min – rinsed – sterile water – remove residual disinfectants
Eg: Mercuric chloride (0.1- 1 %) - 2-10 min
Silver nitrate (1%) - 5-30 min
Bromine solution (1-2%) – 2-10 min
Hydrogen peroxide (10-12%) – 5-15 min
Sodium hypochlorite (1-2%) – 5-30 min
Sterilization of seeds
Seeds washed with sterile distilled water à 70 % alcohol, 1 min Sterilizing agent for specific period àWash with sterile distilled water àmoist cotton/filter paper àgerminated part transferred
Sterilization of other vegetative part
Explants washed with sterile distilled water à 70 % alcohol, 1 min à Sterilizing agent for specific period à Wash with sterile distilled water àtransferred to the media
Types - Plant Tissue Culture Techniques
1. Organ culture or differentiated tissue culture
Excised root tip culture
Excised meristem culture
Embryo culture
Leaf primordia culture
2. Undifferentiated tissue culture in solid media or callus culture
3. Undifferentiated tissue culture in liquid media
Suspension culture
Protoplast culture
Pollen culture
Callus culture
• Callus – mass of undifferentiated unorganised parenchymatous cells proliferate on explant - grown in solid medium
• Each callus cell – has all the genetic information - similar to parent cells – under suitable condition – give rise to new plant
• Addition of suitable growth hormones à Differentiate into root and shoot system àEntire seedlings
• Possible to produce secondary metabolites by adding precursors
Technique of Callus culture:
• Selection, cleaning and sterilization of apparatus
• Selection and preparation of media
• Selection and sterilization of explant
• Establishment of culture / Inoculation of media
• Growth measurement
1. Selection, cleaning and sterilization of apparatus
• Callus is three dimensional / irregular growth
• Petri plates are not suitable, conical flasks or culture tubes made of glass or plastic are suitable
• Glass apparatus – Borosilicate glass, plastic apparatus – High quality plastic
• To close the containers nonabsorbent cotton/ silicone rubber/ aluminium foil should be used
• Apparatus must be cleaned using detergents (soap water teapol 1 hr), conc. HCl- 2 hrs - remove traces – washed with sterile distilled water
• Cleaned glass apparatus sterilized by dry heat sterilization / hot air oven - 140-160˚C – 2 hrs - Plastic apparatus do not require sterilization
2. Selection and preparation of media
• Media should be selected in such a way that it should provide maximum growth with less nutrients
• Media - auxins – high level – induce callus formation + cytokinins
3. Selection and sterilization of explant
• Explant – buds, leaves, stem, roots, seeds, embryos, cotyledons, anthers, pollens and hypocotyls
4. Inoculation of explant/Establishment of culture
• Sterilized explant is transferred to medium under aseptic conditions
• Care must be taken i.e, it must not be submerged inside the media
• Incubate at suitable temperature (25-35 0C)
• Light - Aerial parts – Fluorescent light, subterranean parts – Dark condition
• Acidity – medium – PH – 5.5 – 6.0 – depends upon the strain of tissues employed
• Callus initiation occurs within 2-3 weeks, reaches maximum growth after 5-6 weeks
• Growth curve of callus involves Lag phase, Steady phase, Exponential phase and Decline/death phase
Callus culture Growth Curve:
• Lag phase:Inoculation of explant - Lag time before the cells undergo cell division - Slow growth
• Exponential phase:Vigorous growth and rapid cell division – tissues consume nutrients – media – deplete
• Decline phase: Depletion of elements – media – starvation of cells – decline in the growth of cells
Subculture: Decline phase of cells can be prevented by sub culturing
• Process in which a small piece of callus is transferred to a fresh medium for maintaining steady growth
5. Growth measurement
a. Determination of fresh wt/dry wt of callus
• Callus cells are collected on pre weighed nylon membrane and weighed, gives fresh wt
• Collected cells dried in oven at 80 0C and weighed to a constant weight, gives dry wt
b. Cell viability
• Capacity of a cell or an organism to live and grow
• Rather than growth measurement – metabolic activity is measured cells exhibit green fluorescence under UV light
c. Determination of cell count/cell number
• Gives accurate information – cell growth
• Disruption of clumps – single cell - 1% pectinase or macerating fluid, consists equal volume of 10% chromic acid and 10% nitric acid before counting
• Separated cells are stained with suitable staining reagent and counted on cell counting chamber – Determined using haemocytometre
• Expressed as number of cells per original volume of culture
Callus Culture from tap root of Carrot
Summary
• Tissue culture is growing of tissues or cells on a suitable nutrient medium under aseptic conditions in vitro, may be plant or animal tissue culture
• Types include callus, suspension, hairy root, single cell, haploid, organogenesis, embryo culture etc
• Callus is also called as biomass, grown on semi solid media, mass of undifferentiated parenchymatous cells
• Technique include selection and sterilization of apparatus, media, and explant, establishment of culture and growth measurement







0 Comments: