
Para sympathomimetic agents - Medicinal Chemistry
Para sympathomimetic agents
Intended learning outcomes
At the end of the lecture students will be able to
• SAR of Para sympathomimetic agents
• Classify cholinergic drugs
• Chemical structure, medicinal uses of classified cholinergic drugs
• Outline the synthesis of Carbachol
• Write chemical structure, medicinal uses of Indirect acting/ Cholinesterase inhibitors (Reversible & Irreversible)
• Outline the synthesis of Neostigmine
• Cholinesterase reactivator: Pralidoxime chloride
Contents
• SAR of Para sympathomimetic agents
• Classify cholinergic drugs
• Drug profile of cholinergic drugs
• Outline the synthesis of Carbachol
• Drug profile of indirect acting/ Cholinesterase inhibitors (Reversible & Irreversible)
• Outline the synthesis of Neostigmine
• Cholinesterase reactivator: Pralidoxime chloride
SAR of Para sympathomimetic agents
Onium Group
• Essential for affinity and intrinsic activity
• It interact with the –vely charged Aspartic acid residue of the receptor
• Trimethylammonium group is optimal function moiety. (Exception is pilocarpine, arecoline, nicotine)
• Substitution with larger alkyl groups decrease the activity.
Ester Group
• Essential for affinity, forms H bond with threonine and asparagine residue at the receptor site.
• When methyl replaced by higher homologues (i.e., the propionyl group), the resulting esters are less potent the Ach.
• Aromatic Group possess cholinergic antagonist activity
• NH2 Group (carbamic acid ester group)
• It is more stable than carboxylate esters to hydrolysis.
Ethylene Bridge
• Shortening or lengthening of ethylene bridge decrease M activity
• α substation decrease both M (in greater extent) and N Activity.
• β substation decrease both M (in greater extent) and N Activity.
Classification of Para sympathomimetic agents
Para sympathomimetic agents
Direct-acting cholinergic drugs:
There are two classes of the drugs:
(A) Choline esters and
(B) Cholinomimetic alkaloids
Mechanism of Action of Directly Acting Cholinergic Drugs
• These drugs mediate the actions through muscarinic and nicotinic receptor subtypes.
• Stimulation of M1orM3 receptors causes hydrolysis of polyphosphoinositides and mobilization of intracellular Ca2+, as a consequence of interaction with a G protein and phospholipase C is activated, which phosphorylates the target protein.
• In contrast, M2 and M4 inhibit adenylcyclase and regulate specific ion channels, that is, enhancement of K+ conductance in cardiac arterial tissue.
• Cholinergic stimulation affects cardiac function directly by inhibiting the effects of adrenergic activation.
• As a part, it decreases the cAMP formation and reduction on L-Type Ca2+ channel activity. In arterial muscles, acetylcholine decreases the strength of contraction.
• This effect is due to M2 receptor mediated action of G protein regulated K+ channels. Increased K+ permeability leads to hyperpolarization and shortens the duration of action potentials.
Para sympathomimetic agents
Direct-acting cholinergic drugs
i. Acetylcholine chloride (Miochol)
Properties:
• It is a white or almost white crystalline powder or colourless crystals, very hygroscopic in nature, slightly soluble in methylene chloride, soluble in water and alcohol.
Medicinal uses:
• It is a topical ophthalmic drug to induce miosis, during certain intraocular surgical procedures, such as cataract surgery, ridectomy, penetrating keratoplasty, and other anterior-segment surgery.
• Systemically administered Ach is rapidly hydrolyzed by acetylcholinesterase, hence, it has no clinical use.
• It is a cardiac depressant and effective vasodilator.
ii. Methacholine chloride (Provocholine)
Properties: It is highly deliquescent, has faintly shy odour, and aqueous solutions are neutral, soluble in water, alcohol, and CHCl3.
Medicinal uses:
• It is used to treat Reynaud’s syndrome and glaucoma
• Methacholine was used in the past to control supraventricular tachycardia and is replaced with ectrophonium and other drugs, which are safer
• Methacholine is also used for diagnosis of belladonna (i.e., muscarinic antagonist) poisoning
• For diagnosis of familial dysautonomia, and for diagnosis of bronchial hyper reactivity (i.e., supersensitivity to bronchoconstriction in patients with asthma)
Cholinomimetic Alkaloids:
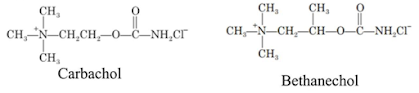
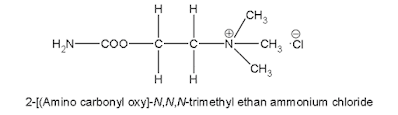
i. Carbachol
Properties:
• It is a white crystalline, hygroscopic powder, soluble in water, sparingly soluble in alcohol, insoluble in acetone.
• It is an ester of carbamic acid, the terminal methyl group of Ach is replaced by amino group.
Medicinal uses:
• It possesses both muscarinic and nicotinic properties by cholinergic receptor stimulation
• It is more slowly hydrolyzed by acetylcholinesterase
• It is used for its miotic actions in the treatment of glaucoma to reduce intraocular pressure
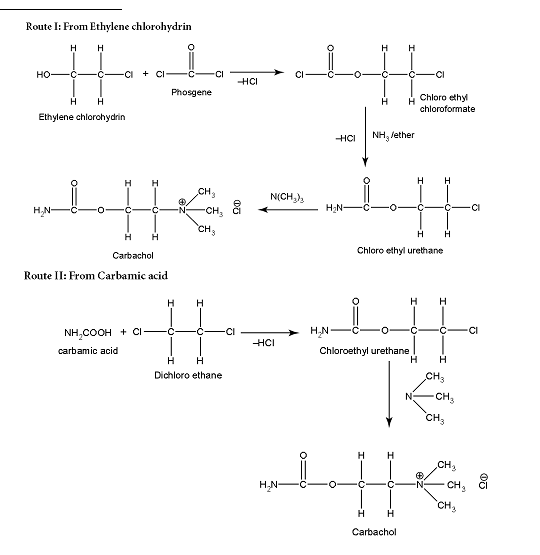
Carbachol Synthesis
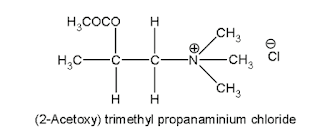
ii. Bethanechol chloride
(Synonym: Urecholine, Myotonachol, Bethacol, Urotonin)
Properties: It is a white crystalline hygroscopic powder, and it exhibits polymorphism, soluble in water and alcohol.
Medicinal uses:
• It has pharmacological properties similar to those of methacholine.
The presence of –CH3 gives prolonged activity due to steric hindrance.
• It produces smooth muscle contractions.
• It can be given subcutaneously, but not by intramuscular (IM) or intravenous (IV) because of its severe side effects.
• It is used to relieve urinary retention and abdominal distention after surgery. This is one of the postvagotomy gastric drug
iii. Pilocarpine
Properties and uses:
• It is a white or almost white crystalline powder or colourless crystals, hygroscopic,very soluble in water and in alcohol.
• Pilocarpine is an alkaloid obtained from the dried leaflets of Pilocarpus jaborandi and Pilocarpus microphyllus in which it occurs to the extent of about 0.5% together with other alkaloids.
• Chemically it is 3-ethyldihydro-4[(1-methyl-1H-imidazol-5-yl)- methyl] furan-2(3H)-one
• Pilocarpine is a nonselective agonist on the muscarinic receptors.
• It acts on M3 receptors in smooth muscles and cause contractions in the gut, trachea, and eyes.
• It is used for the treatment of symptoms of dry mouth caused by radiotherapy for cancer of head and neck and the symptoms associated with Sjogren’s syndrome.
• Mostly used as a solution (1 to 5%) to exert an action on the eye to cause miosis and retard intraocular tension in the treatment of open-angle glaucoma
Indirect Acting Cholinomimetic Drugs
• The actions of acetylcholine released from autonomic and somatic motor nerves are terminated by enzymatic destruction of the molecule
• Hydrolysis is accomplished by acetylcholinesterase
• The indirect acting drugs have primary effect on the active site of this enzyme, although some also have direct actions at nicotinic receptors
• The common differences between members of the group are chemical and pharmacokinetic, but their pharmacodynamics properties are identical
Mechanism of Action of Indirectly Acting Cholinergic Drugs
(Anticholinesterase Agents)
• Acetylcholinesterase (AchE) is a serine dependent isoenzyme capable of hydrolyzing Ach to choline and acetic acid.
• The active site of AchE comprises two distinct regions, an anionic site that possess a glutamate residue and an esteratic site in which histidine imidazole ring and serine –OH group are present.
• Catalytic hydrolysis occurs, thereby the acetyl group is transferred to the serine –OH group, leaving an acetylated enzyme molecule and a molecule of free choline
• Spontaneous hydrolysis of the serine acetyl group occurs rapidly
There are two main categories of AchE inhibitors:
1. The amine or ammonium AchE inhibitors react reversibly with enzymes, these compounds reversibly acylate the esteratic serine hydroxyl, their duration of action are few minutes to few hours.
2. The organophosphate type AchE inhibitors form an irreversible fi rm bond with the enzymes (esteratic site) and their duration of action are few weeks to months.
A. Reversible blockers
i. Physostigmine (Isopto-Eserine):
Properties:
• It exists as a white or almost white crystalline powder, hygroscopic, very soluble in water, and freely soluble in alcohol. It gradually becomes red when exposed to air and light; the colour develops more quickly when the substance is also exposed to moisture.
• Aqueous solutions are unstable. It melts at about 145°C with decomposition.
• It is an alkaloid obtained from the dried ripe seeds of Physostigma venenosum.
Medicinal uses:
• Physostigmine is an oldest anticholinesterase agent
• It is used in the treatment of glaucoma
• It can penetrate the blood brain barrier and is employed to antagonize the toxic CNS effects of antimuscarinic drugs, tricyclic depressants, H1 antihistamines, and benzodiazepines
• It is also used in the treatment of Alzheimer’s disease
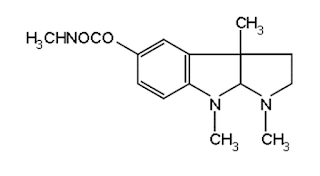
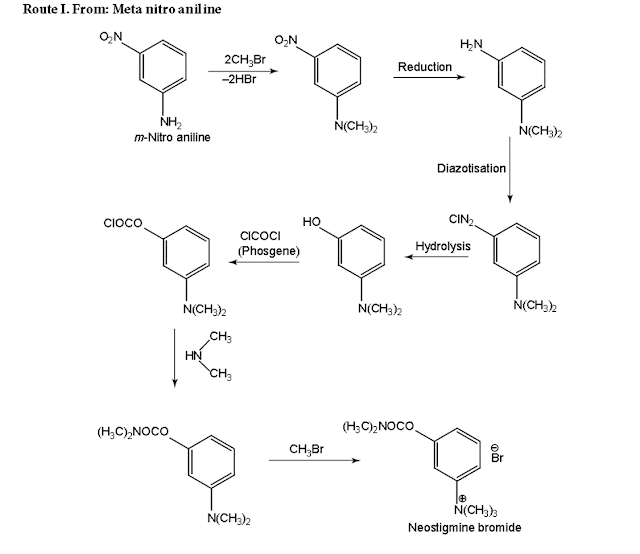
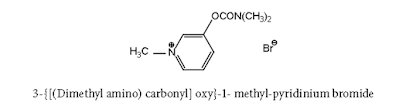
ii. Neostigmine Bromide
(Synonym: Prostigmine, Myostigmin, Tilstigmin)
Properties: It exists as white, odourless, crystalline powder with a bitter taste, freely soluble in water, alcohol, and insoluble in ether. Its solutions are neutral to litmus.
Medicinal uses:
It acts as a cholinesterase inhibitor.
Neostigmine Bromide synthesis
iii. Pyridostigmine Bromide
(Synonym: Mestion, Pyrido, Trostigmin)
Properties: It exists as white, crystalline powder with a characteristic odour and bitter taste, soluble in water, alcohol, chloroform, slightly soluble in hexane, and insoluble in ether. It is hygroscopic in nature.
Medicinal uses:
It is used in the treatment of myasthenia gravis and it antagonizes the effects of neuromuscular blocking (NMB) agents
iv. Edrophonium Chloride (Tensilon)
Properties: It exists as a white crystalline powder, soluble in water and alcohol, insoluble in methylene chloride. On parenteral administration, edrophonium has a more rapid onset and shorter duration of action than neostigmine, pyridostigmine, or ambenonium.
Mechanism of action of Edrophonium Chloride:
Quaternary ammonium compounds inhibit the enzyme reversibly by either binding with the esteratic site, or with a site spatially removed, termed the peripheral anionic site
Medicinal uses:
• It is used as an antiarrhythmic drug in paroxysmal atrial tachycardia.
• It is also used in the diagnosis of myasthenia gravis
v. Tacrine Hydrochloride
• is the hydrochloride salt form of tacrine, an aminoacridine derivative
Medicinal uses:
• Tacrine has been used to counter the effects of muscle relaxants
• As a respiratory stimulant
• In the treatment of Alzheimer's disease
vi. Ambenonium Hydrochloride
• Ambenonium is a bisquaternary ammonium alcohol with parasympathomimetic activity
• It acts by suppressing the activity of acetylcholinesterase
Chemically it is [oxalylbis(iminoethylene)] bis[(ochlorobenzyl)diethylammonium]dichloride.
Properties: It is white, odourless, water soluble solid
Medicinal Uses: Cholinesterase Inhibitor
• Ambenonium is used to treat myasthenia gravis.
B. Irreversible inhibitors
i. Echothiopate
Echothiopate is an organophosphate available as echothiopate iodide.
Properties:
• Echothiopate iodide is white, crystalline characteristic odor, (mercaptan like odous) hygroscopic powder. It is soluble in water.
Medicinal Uses:
• Echothiopate is a long acting irreversible anti-AChE drug that is used in the treatment of glaucoma.
ii. Malathion
• Malathion is another effective pesticide, which is more effective on insects than on humans because it requires biotransformation to the phosphate form, which can only be carried out by insects..
• Chemically it is 2- [(dimethoxyphosphinothioyl)thio]-butanedioic acid diethyl ester. Malathion is a phosphodithioate ester
Properties: Malathion is available as a light amber colored liquid having sulphur like odour
Medicinal uses:
• It is poor irreversible inhibitor of choline esterase enzyme
• Malathion is used extensively for controlling insects on vegetables, fruits, and cereal crops.
• It is also used for controlling insects affecting man and animals
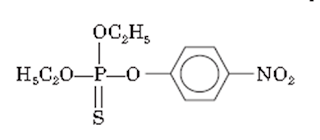
iii. Parathion
• Parathion is O, O-diethyl O-p-nitrophenyl phosphorothioate.
• It is a weak cholinesterase inhibitor.
Properties:
• Parathion occurs as a brown colored liquid
Medicinal uses:
• Parathion is used as an agricultural insecticide. It is especially used for controlling aphids, spider mites, and scale insects
iv. Isofluorphate
• Isofluorphate is an organophosphate. Isofluorphate covalently binds to acetylcholinesterase and inhibits acetyl cholinesterase irreversibly.
Properties:
• Isofluorphate is a colorless, water-miscible liquid.
Medicinal Uses:
• Isoflurophate is used to treat glaucoma.
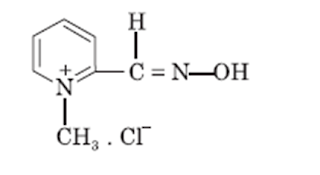
Cholinesterase reactivator:
Pralidoxime chloride:
• Pralidoxime is an aldoxime and available as pralidoxime chloride.
• Chemically it is 2-formyl-1-methylpyridinium chloride oxime
Properties:
• Pralidoxime chloride is colorless or light yellow colored, water soluble, and crystalline powder.
Medicinal Uses:
• The molecule pralidoxime is a useful antidote for intoxication with cholinesterase inhibitors such as the organophosphates.
• The molecule removes the inhibitor from the active site in the form of an oxime phosphonate


















0 Comments: