
Anti psychotics Agents - Medicinal Chemistry
ANTIPSYCHOTICS AGENTS
Intended learning outcomes
At the end of this lecture, student will be able to:
• Define psychotropic drugs
• Categorize the psychotropic agents
• Describe the SAR of phenothiazines
• Discuss about some antipsychotic agents
• Outline the synthesis of some antipsychotic agents
Contents
• Psychotropic drugs
• Categorize the psychotropic agents
• The SAR of Phenothiazine
• Important drugs used as antipsychotic agents
• The synthesis of some antipsychotic agents
Psychotropic agents/Tranquilizers
• Psychoactive or psychotropic drugs - also known as tranquilizers
• These drugs are used in the treatment of psychiatric disorders i.e. abnormalities of mental function
• The psychoactive drugs render the patient calm and peaceful by reducing agitation and anxiety
• They cause sedation without inducing sleep
• Psychoactive drugs does not cure mental disorders, control most symptomatic manifestations and behavioral deviances
• Tranquilizers are drugs essentially used in the management and, treatment of psychoses and neuroses
• They specifically exert their action on the lower brain areas to produce emotional calmness and relaxation without appreciable hypnosis sedation euphoria or motor impairment
• In addition many of these drugs also display clinically beneficial actions, for instance skeletal muscle relaxants, antihypertensive, antiemetic and antiepileptic properties
• Major tranquilizers and minor tranquilizers have overlapping characteristic features
• Anti-psychotic drugs are used to treat psychoses like schizophrenia, mania, senile dementia and behavior disorders in children
• These drugs act by depressing the central nervous system (by decreasing dopamine levels)
• They produce sedation without producing sleep
• Their overall influence is to free the mind from disturbance and thus calm the mind
• They reduce excitation, agitation, aggressiveness and impulsiveness
• Drugs which better mental aberrations that are invariably characteristic feature of psychoses
Psychotropic Agents- Classification
• The primary characteristic feature of these drugs is that they alter the mental state and behavior in a predictable way.
• The psychoactive drugs are classified as;
1. Antipsychotic drugs - Major tranquilizers (for psychoses)
2. Anti-depressant drugs - Minor tranquilizers (for neuroses)
3. Anti-anxiety drugs
Psychoses-Symptoms
• Positive symptomsof psychoses essentially comprise of a host of disorders, such as: mild behavioral changes anxiety, delusions, hallucinations, and schizophrenias.
• Negative symptomsare usually designated by cognitive deficits, social withdrawal, apathy, and anhedonia
Psychoses- cardinal requirements
The typical ‘antipsychotics’ should ideally possess the following cardinal requirements, such as:
• High lipid solubility
• Affinity for protein-binding (92–99%),
• Large volume of distribution (vd 55) i.e., greater than 7 L.kg–1,
• Variance in oral bioavailability (25–35%), and
• Short plasma half-life between 10–20 hours
Classification of antipsychotic agents
The drugs used in the treatment of psychoses are classified as follows:
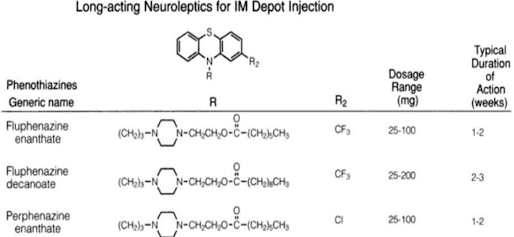
1. Phenothiazine derivatives: Promazine hydrochloride, Chlorpromazine hydrochloride*,Triflupromazine, Thioridazine hydrochloride, Piperacetazine hydrochloride, Prochlorperazine maleate, Trifluoperazine hydrochloride
2. Fluro butyrophenones: Haloperidol, Droperidol, Risperidone
3. Dihydroindolones/Beta amino ketones: Molindone hydrochloride
4. Thioxanthines/Ring Analogues of Phenothiazines: Chlorprothixene, Thiothixene, Loxapine succinate, Clozapine
5. Benzamides:Sulpieride
Phenothiazines
• Phenothiazines act exclusively on specific postsynaptic receptors and block the postsynaptic dopamine receptors.
They work on the positive symptoms of psychosis such as hallucinations, delusions, disorganized speech, looseness of association, and bizarre behavior.
• The following is the general structure of phenothiazines
SAR OF PHENOTHIAZINES
The neuroleptic properties of phenothiazine may be affected by following:
• Nature of the chain in the position N10
• Nature of the amino group
• Substitution at second position
• Replacement of the H in position 2
• Substitution at position 3
• Substitution at position 1
• Three carbon chain
• Branching with larger groups
• Replacement of terminal alkly amino group
1. Unsubstituted Phenothiazines has no activity but has enough lipophilicity for good brain penetration
• Substitution at C2 and N10 is required for activtiy
2. Potency increases in the following order of position of ring substituents
1˂4˂3˂2
C2 must have an electron withdrawing group
The activity for these various group is as
X = - SO2NR2 > -CF3 > -CO-CH3 > -Cl
Ex: Chlorpromazine (chlorine)
3) A terminal amino substituent must be present at N10.
It can be piperazine, piperidine or aliphatic and their intensity could be ranked as follows: piperazine group >piperidine group > aliphatic chain
A piperidine ring in the side chain show lower incidence of extrapyramidal side effects (e.g. Parkinsonism) possibly due to increased central antimuscarinic activity. E.g. thioridazine.
• Esterification of the OH containing piperazine derivatives extensively increases the duration of action, less hypotension
4. Alkyl Side chain:There must be an linear (ie unbranched) alkyl linker between the core ring and the terminal amino ring those length is optimum at three methylene units ie CH2-CH2-CH2
Reduction of these carbon number changes receptor affinity
• Branching at the β position of the side chain with small methyl group – DECREASE IN ACTIVITY.
• β position substitution with larger group - LOSS IN ACTIVITY. Bridging of position 3 of the side chain to position 1 – LOSS IN ACTIVITY
Phenothiazine ring:
• Oxidation of 5 sulphur: decrease activity
• 1,2,3,4 aza phenothiazine: more potent
For example: 1-aza analogue of promazine is more potent than parent compound
• If nitrogen is replaced by carbon then the compound thioxanthine is produced which is also active but less potent compared to phenothiazine
• Phenothiazinesexert their antipsychotic potency by interacting with a receptor at three marked sites X, Y, Z to produce a singificant response.
– The order of specific structural requirement at these sites is YZX. However, a three-carbon chain at site Y affords an optimal antipsychotic activity
Promazine Hydrochloride
• Promazine is a phenothiazine derivative
• Chemically it is 10-[3-(dimethyl amino)-propyl] phenothiazine
Properties
• Promazine is available as hydrochloride salt. Promazine HCl is white or slightly yellow crystalline powder, and is freely soluble in water and chloroform. It should be protected from air.
Medicinal Uses
• Promazine has antipsychotic properties: dopamine receptor antagonist and neuroleptic
• It is also used to control nausea and vomiting
• An older medication used to treat schizophrenia
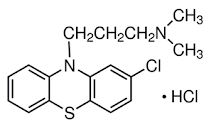
Chlorpromazine hydrochloride INN, BAN, USAN
• Chlorpromazine hydrochloride is a phenothiazine derivative and
• Chemically it is 2-chloro-10-[3-(-dimethylamino) propyl] phenothiazine monohydrochloride
Properties
• Chlorpromazine hydrochloride is an odorless, white crystalline powder. It is freely soluble in water, alcohol, chloroform and insoluble in ether and benzene. It decomposes on exposure to air and light hence it should be stored in airtight containers and protect from light
Medicinal Uses
• Chlorpromazine is used in the management of psychotic conditions. It also controls excitement, aggression and agitation.
• It has antiemetic, antipruritic, anti-histaminic and sedative properties
Chlorpromazine hydrochloride Mechanism of Action:
• Exact mechanism is not absolutely determined. Chlorpromazine is thought to block dopamine at D2 receptor sites in the mesolimbic medullary chemoreceptor trigger zone areas of the brain.
• It causes inhibitory post-synaptic effects by reducing the flow of dopamine as the dopaminergic ion channels are closed
Chlorpromazine hydrochloride Synthesis:
Step I:
• Chlorpromazine is synthesized by cyclization of 3- chloro diphenylamine with sulphur in presence of small amount of iodine as catalyst
Step II:
• Refluxing a toluene solution of 2-chlorophenothiazine and 3-chloropropyl dimethylamine in the presence of sodamide for several hours, followed by filtration and removal of toluene under reduced pressure.
Triflupromazine
• Triflupromazine is a fluorinated phenothiazine derivative.
• Chemically triflupromazine is 10-[3-(dimethylamino)propyl]-2-(trifluoromethyl) phenothiazine
Properties:
• Triflupromazine occurs as hydrochloride salt. Triflupromazine hydrochloride is white, crystalline powder. It is freely soluble in water, alcohol and insoluble in ether.
Medicinal Uses:
• Triflupromazine is used to treat psychotic disorders
• It also has antiemetic properties
Thioridazine hydrochloride
Properties: It is a white or almost white crystalline powder, soluble in ethanol, freely soluble in water and in methanol.
Medicinal uses:
• The drug has sedative and hypotensive activity in common with chlorpromazine.
• It is effective in the management and manifestations of psychotic disorders, used as Dopamine receptor antagonist and neuroleptic
• The drug exerts minimum antiemetic activity and there by gives rise to minimal extrapyramidal stimulation
Piperacetazine hydrochloride
• Chemically it is 1-(10-{3-[4-(2-Hydroxyethyl)-1-piperidinyl]propyl}-10H-phenothiazin-2-yl)ethanone hydrochloride
• It is an antipsychotic prodrug, most notably used for schizophrenia
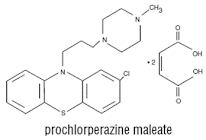
Prochlorperazine maleate
Properties: It is a white or pale yellow crystalline powder, well soluble in water and alcohol.
• It is a dopamine (D2) receptor antagonist that belongs to the phenothiazine class of antipsychotic agents
Medicinal uses:
• For the antiemetic treatment of nausea and vertigo
• It is also a highly potent typical antipsychotic, 10–20 times more potent than chlorpromazine
• It is also used to treat migraine headaches
• It is used to prevent vomiting caused by chemotherapy, radiation therapy and in the pre- and postoperative setting
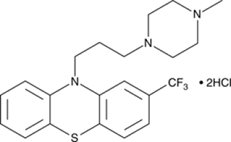
Trifluoperazine hydrochloride.
• Chemically it is 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine, dihydrochloride
• Trifluoperazine (TFP) is a phenothiazine compound with anti-adrenergic and anti-dopaminergic actions typical of antipsychotic agents
Thioxanthines/Ring Analogues of Phenothiazines:
Chlorprothixene
Thiothixene
Loxapine succinate
Clozapine
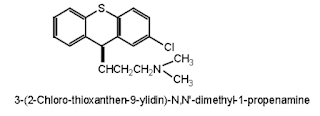
Chlorprothixene
• Chlorprothixene is a typical antipsychotic drug of the thioxanthene (tricyclic) class.
• Chemically it is (3Z)-3-(2-Chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine
Properties: It is a white or almost white crystalline powder, slightly soluble in methylene chloride, and soluble in water and alcohol.
Medicinal uses
• It is used in the treatment of acute and chronic schizophrenia, psychotic and other conditions in which anxiety, agitation, and tension predominate.
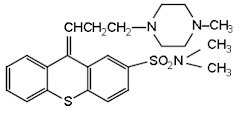
Thiothixene
• Thiothixene is a thioxanthine used as an antipsychotic agent. Its effects are similar to the phenothiazine antipsychotics.
• Chemically it is (9Z)-N,N-dimethyl-9-[3-(4-methylpiperazin-1- l)propylidene]thioxanthene-2-sulfonamide
Properties:White, or nearly white crystalline powder with slight odour, and affected bylight, soluble in water, anhydrous alcohol, or chloroform, practically insoluble in benzene, acetone, orether. The substituent in the second position produces Z and E isomers. The Z isomers are the moreactive antipsychotic isomers.
Medicinal uses
• It was introduced as an antipsychotic agent useful in the management of schizophrenia and other psychotic states.
• It is also helpful in the management of secondary symptoms of schizophrenia, such as hallucinations, tension, and suspiciousness.
• It also shows antidepressant property.
Loxapine succinate
• Loxapine succinate is an antipsychotic agent used in schizophrenia.
Medicinal uses:
• D2/D4 dopamine receptor antagonist
• 5-HT2A/2B, 5-H7 serotonin receptor antagonist
• Dibenzoxazepine antipsychotic agent
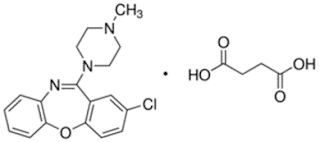
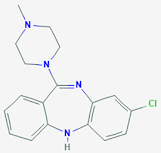
Clozapine
• Clozapine is a tricylic dibenzodiazepine, classified as an atypical antipsychotic agent.
• It binds several types of central nervous system receptors, and displays a unique pharmacological profile.
• It may be better than other antipsychotics in people with both schizophrenia and Parkinson's disease
• Chemically it is 3-chloro-6-(4-methylpiperazin-1-yl)-11H-benzo[b][1,4]benzodiazepine
Fluro butyrophenones:
Haloperidol
Droperidol
Risperidone
Fluro butyrophenones:
Haloperidol INN, BAN, USAN:
• Haloperidol is a butyrophenone
Properties: White to faintly yellowish, practically insoluble in water, soluble in methanol-acetone, benzene,
Medicinal uses
• It is useful in the management of psychotic reactions, hostility, and hyperactivity.
• It is a drug of choice for Tourett’s syndrome.
• Haloperidol is an effective neuroleptic
• Antiemetic properties
Droperidol:
• Droperidol is butyrophenone derivative used as sedative, tranquilizer, and anti-nausea medicine
Properties and uses:It is a white or almost white powder insoluble in water, sparingly soluble in alcohol, freely soluble in dimethyl formamide and in methylene chloride.
Medicinal Uses:
• It is frequently used in combination with the nacrotic agents pre-anaesthetically.
• It is a neuroleptic used as an adjunct to anaesthesia to produce sedation and reduce incidence of nausea and vomiting.
• Also used as β1-adrenoceptor agonist α-adrenoceptor agonist
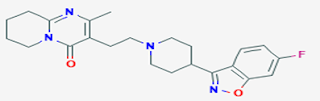
Risperidone:
• benzisoxazole derivative or flurobutyrophenones
• Chemically it is 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
Properties:
• Risperidone is a white to slightly beige powder.
• It is practically insoluble in water, freely soluble in methylene chloride, and soluble in methanol and 0.1 N HCl
Mechanism of action of Risperidone:
• It has been proposed that the drug's therapeutic activity in schizophrenia is mediated through a combination of dopamine Type 2 (D2) and serotonin Type 2 (5HT2) receptor antagonism
Medicinal uses:
• It is a medication known as an atypical antipsychotic that is used to treat symptoms of schizophrenia in teenagers and adults.
• Mood disorders, including bipolar disorder and depression with pyschosis
Beta amino ketones/Dihydro indolones:
Molindone hydrochloride
Properties:Exists as white crystals, freely soluble in water or alcohol,
Medicinal uses:
• It is a potent antipsychotic as trifluoperazine and all the side effects resemble those of the phenothiazines.
• It is used in the treatment of schizophrenia and other psychosis
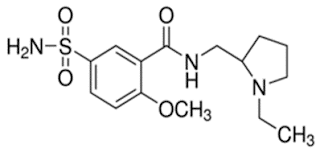
Benzamides: Sulpieride
• Sulpiride is a substituted benzamide derivative
• Chemically it is N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxy-5-sulfamoylbenzamide
• A dopamine D2-receptor antagonist
• It has been used therapeutically as an antidepressant, antipsychotic, and as a digestive aid.
Summary
• These drugs are used in the treatment of psychiatric disorders i.e. abnormalities of mental function.
• The psychoactive drugs render the patient calm and peaceful by reducing agitation and anxiety
• Phenothiazines exert their antipsychotic potency by interacting with a receptor at three sites to produce a singificant response

















0 Comments: