
Respiratory chain, its role in energy capture and its control
Respiratory chain, its role in energy capture and its control
Objective
• At the end of this lecture, student will be able to
– Explain Respiratory chain
– Describe structural organisation of ETC
– Discuss components of ETC
– Discuss Inhibitor of ETC
Electron Transport Chain
• The energy rich carbohydrates, fatty acid and amino acid undergo a series of metabolic reaction and are finally oxidized to CO2 and H2O
• The reducing equivalents from various metabolic intermediates are transferred to coenzymes NAD+and FAD to produce NADH & FADH2
• The latter two reduced coenzymes pass through ETC and finally, reduce oxygen to water
• The passage of electrons through the ETC is associated with the loss of free energy
• A part of this free energy is utilized to generate ATP from ADP and Pi
Mitochondria: The power houses of cell
• Mitochondria are the centres for metabolic oxidative reactions to generate reduced co-enzymes (NADH & FADH2) which are utilized in ETC to liberate energy in the form of ATP. Hence, regarded as the power house of the cell
• It consists of 5 distinct parts, outer membrane, inner membrane, inter-membrane space, cristae and matrix
• ETC & ATP synthesizing system are located on the inner mitochondrial membrane, which is a specialized structure, rich in proteins. It is impermeable to ions (H+, K+, Na+) and small molecules (ADP, ATP)
• This membrane is highly folded to form cristae
– increases the inner surface area
• The inner surface consist of phosphorylating subunits which are the centres for ATP production
• Matrix is rich in enzymes responsible for the citric acid cycle, β-oxidation of fatty acids and oxidation of amino acids
Structural organization of respiratory chain
• The inner mitochondrial membrane consist of five distinct respiratory or enzyme complexes, denoted as complex I Il, III, IV and V
• The complexes l-lV are carriers of electrons while complex V is responsible for ATP synthesis
• NADH, coenzyme Q, cytochrome C and oxygen are mobile electron carriers in the respiratory chain
• The enzyme complexes (I-IV) and the mobile carriers are collectively involved in the transport of electrons which, ultimately, combine with oxygen to produce water
• The largest proportion of the oxygen supplied to the body is utilized by the mitochondria for the operation of electron transport chain
Components and reactions of ETC
• Five distinct carriers in ETC
• These carriers are sequentially arranged and are responsible for the transfer of electrons from a given substrate to ultimately combine with proton and oxygen to form water
l. Nicotinamide nucteotides:
• Two coenzymes NAD+ & NADP+ derived from the vitamin niacin, NAD+ is more actively involved in the ETC
• NAD+ is reduced to NADH + H+ by dehydrogenases with the removal of two hydrogen atoms from the substrate (AH2)
e.g. glyceraldehyde-3-phosphate, pyruvate, isocitrate, α-ketoglutarate and maleate
• NADPH + H+ produced by NADP+dependent dehydrogenase is not used in a substrate for ETC. NADPH is more effectively utilized for anabolic reactions (e.g. fatty acid synthesis, cholesterol synthesis)
2. Flavoproteins:
• The enzyme NADH dehydrogenase is a flavoprotein with FMN as the prosthetic group. The coenzyme FMN accepts two electrons and form FMNH2
• NADH dehydrogenase is a complex enzyme closely associated with non-heme iron proteins (NHI) or iron-sulfur proteins (FeS)
• Succinate dehydrogenases is an enzyme found in the inner mitochondrial membrane. lt is also a flavoprotein with FAD as the coenzyme. It can accept two hydrogen atoms from succinate
3. Iron sulfur (FeS) proteins:
• FeS proteins exist in the oxidized (Fe3+) or reduced (Fe2+) state
• One FeS participates in the transfer of electrons from FMN to coenzyme Q
• Other FeS proteins associated with cytochrome b and cytochrome c1 participate in the transport of electrons
4. Coenzyme Q (ubiquinone):
• lt is a quinone derivative with a variable isoprenoid side chain
• The mammalian tissues possess a quinone with 10 isoprenoid units which is known as coenzyme Q10
• Coenzyme Q is a lipophilic electron carrier- lt accepts electrons from FMNH2 produced in the ETC by NADH dehydrogenase
5. Cytochromes:
• The cytochromes are conjugated proteins containing heme group, consists of a porphyrin ring with iron atom
• The iron of heme in cytochromes is alternately oxidized (Fe3+) & reduced (Fe2+), which is essential for the transport of electrons in the ETC
• Three cytochromes were initially discovered from the mammalian mitochondria- designated as cytochrome a, b & c depending on the type of heme present and the respective absorption spectrum
• Additional cytochromes such as c1, b1, b2, a3 etc were discovered later
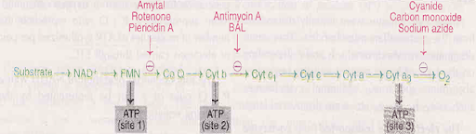
Inhibitors of ETC
• The inhibitors bind to one of the components of ETC and block the transport of electrons & causes the accumulation of reduced components
• The synthesis of ATP is dependent on electron transport. Hence, all the site-specific inhibitors of ETC also inhibit ATP formation
• 3 possible sites
1. NADH and coenzyme Q : Fish poison, rotenone, barbiturate drug amytal and antibiotic piercidin A inhibit this site
2. Between cytochrome b and c1: Antimycin A - an antibiotic, British antilewisite (BAL) –an antidote used against war-gas-are the two important inhibitors of the site between cytochrome b and c1
3. Inhibitors of cytochrome oxidase: Carbon monoxide, cyanide, hydrogen sulphide and azide effectively inhibit cytochrome oxidase
Summary
• The energy rich carbohydrates, fatty acid and amino acid undergo a series of metabolic reaction and finally oxidized to co2 and H2O
• The passage of electrons through the ETC is associated with the loss of free energy and part of this free energy is utilized to generate ATP from ADP and Pi
• Mitochondria are the centres for ETC
• The components of ETC are nicotinamide, Flavoproteins, Iron sulfur proteins, Coenzyme Q
• Fish poison, rotenone, barbituate drug amytal, piercidin A, Antimycin A, British antilewisite (BAL), Carbon monoxide, cyanide, hydrogen sulphide and azide are inhibitors of ETC












0 Comments: